Home FDA PI Contrast Allergy PreMed Pack Prescribing Information
Contrast Allergy PreMed Pack: Package Insert / Prescribing Info
Package insert / product label Generic name: prednisone, diphenhydramineDosage form: kit
Contrast Allergy PreMed Pack Description
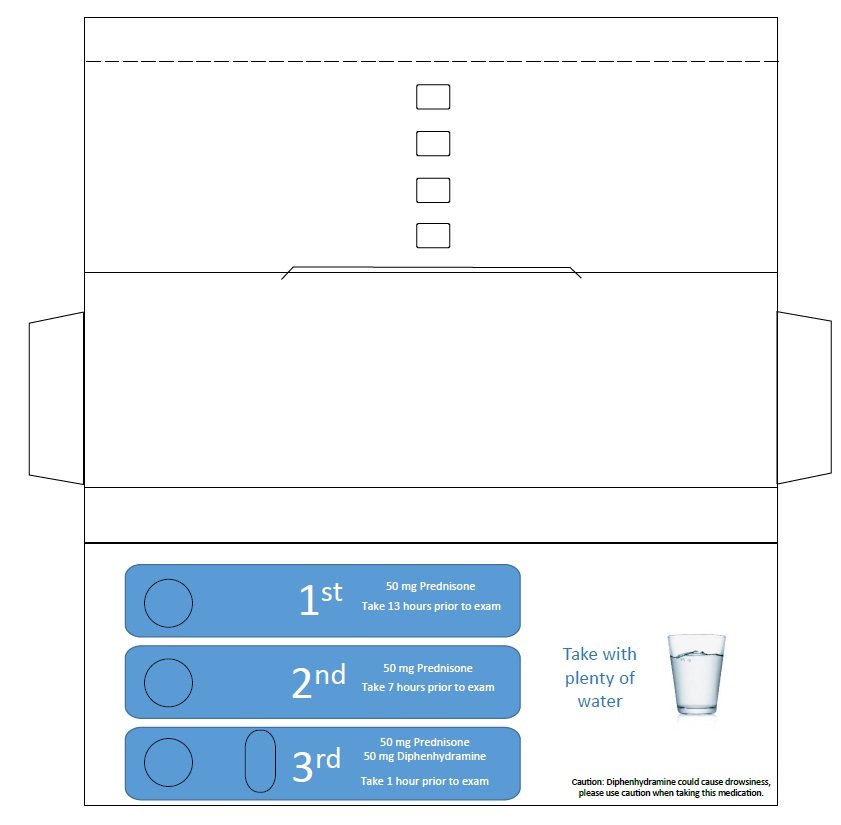
Contrast Allergy PreMed Pack
TM consists of an administration card containing three Prednisone 50 mg tablets, USP, and one Diphenhydramine Hydrochloride 50 mg capsule, USP, for oral administration.
Contrast Allergy PreMed Pack
TM
NDC 16129-101-01
double therapy
Contrast Allergy PreMed Pack
TM
BLISTER PACK CONTAINS:
3 PREDNISONE, USP, 50 mg Tablets; and
1 DIPHENHYDRAMINE HYDROCHLORIDE CAPSULE, USP, 50 mg
Rx only
CONTRAST ALLERGY PREMED PACK
prednisone, diphenhydramine kit
Part 1 of 2
PREDNISONE
prednisone tablet
Part 2 of 2
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsule
Medical Disclaimer