Catosal: Package Insert / Prescribing Info
Package insert / product label
Generic name: enrofloxacin
Dosage form: FOR ANIMAL USE ONLY
On This Page
CAUTION: Federal law (U.S.A.) restricts this drug to use by or on the order of a licensed veterinarian.
Indications and Usage for Catosal
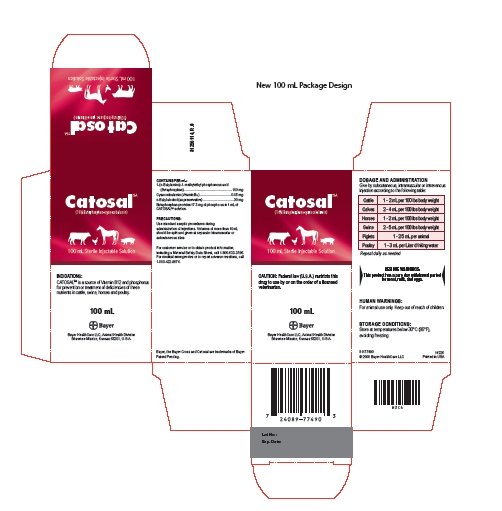
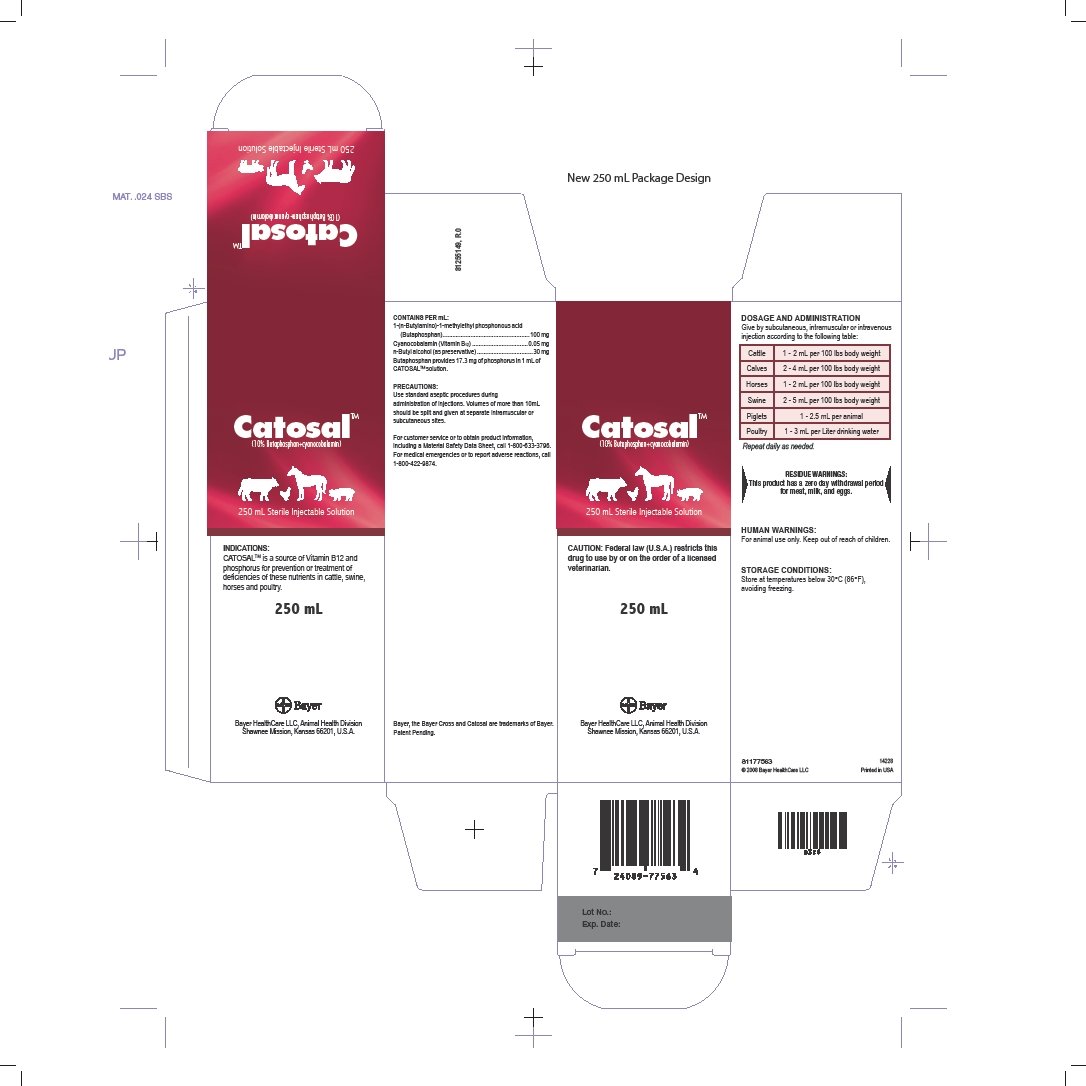
CATOSALTM is a source of Vitamin B12 and phosphorus for prevention or treatment of deficiencies of these nutrients in cattle, swine, horses and poultry.
Contains Per mL:
1-( n-Butylamino)-1-methylethyl phosphonous acid
(Butaphosphan).....100 mg
Cyanocobalamin (Vitamin B12).....0.05 mg
n-Butyl alcohol (as preservative).....30 mg
Butaphosphan provides 17.3 mg of phosphorus in 1 mL of CATOSALTM solution.
Precautions
Use standard aseptic procedures during administration of injections. Volumes of more than 10mL should be split and given at separate intramuscular or subcutaneous sites.
For customer service or to obtain product information, including a Material Safety Data Sheet, call 1-800-633-3796. For medical emergencies or to report adverse reactions, call 1-800-422-9874.
Catosal Dosage and Administration
Give by subcutaneous, intramuscular or intravenous injection according to the following table:
| Repeat daily as needed. | |
| Cattle | 1 - 2 mL per 100 lbs body weight |
| Calves | 2 - 4 mL per 100 lbs body weight |
| Horses | 1 - 2 mL per 100 lbs body weight |
| Swine | 2 - 5 mL per 100 lbs body weight |
| Piglets | 1 - 2.5 mL per animal |
| Poultry | 1 - 3 mL per Liter drinking water |
| CATOSAL
enrofloxacin solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bayer HealthCare LLC Animal Health Division (152266193) |