Home FDA PI AlloPAX Prescribing Information
AlloPAX: Package Insert / Prescribing Info
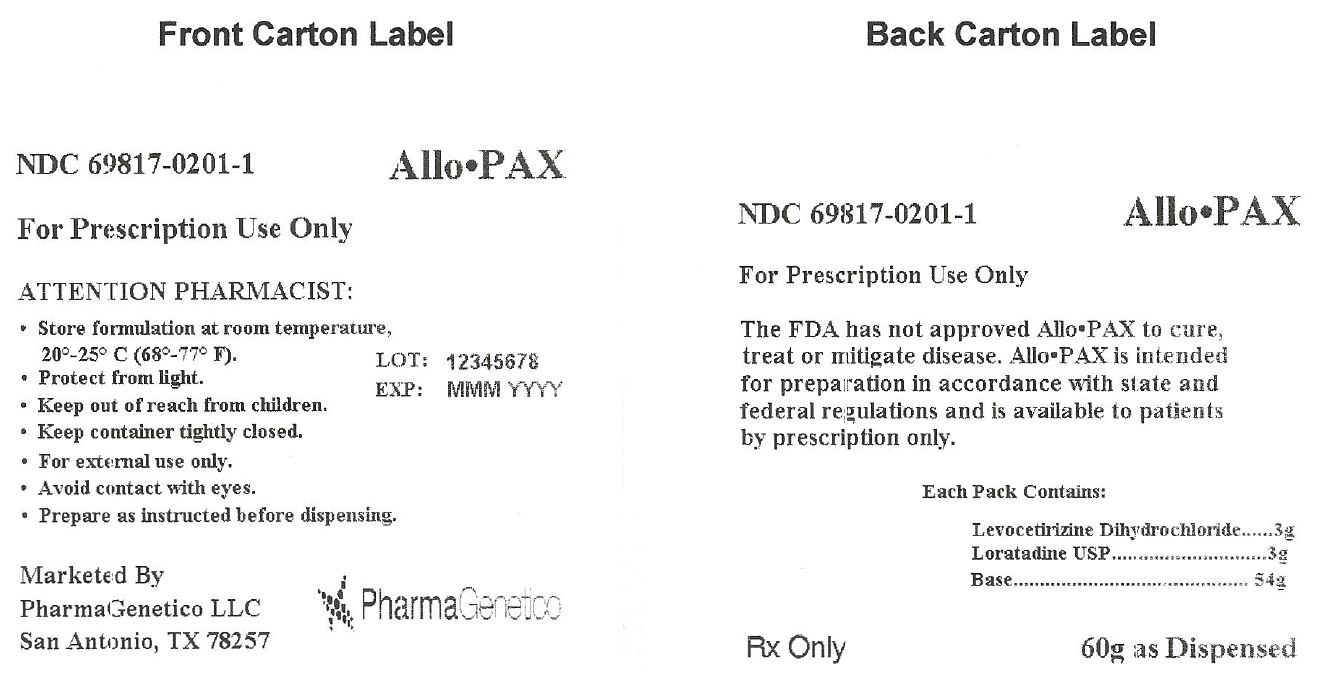
Package insert / product label Generic name: levocetirizine dihydrochloride, loratadineDosage form: topical application
Each Allo•PAX provides 3 grams of Levocetirizine dihydrochloride, 3 grams Loratadine USP, and 54 grams of Base. The resulting mixture is intended for transdermal use.
For Prescription Use Only
Distributed by:
PharmaGenetico LLC
San Antonio, TX 78257
ALLOPAX
levocetirizine dihydrochloride 5%, loratadine 5% kit
Part 1 of 3
LORATADINE
loratadine powder, for suspension
Part 2 of 3
LEVOCETIRIZINE DIHYDROCHLORIDE
levocetirizine dihydrochloride powder, for suspension
Part 3 of 3
CREAM BASE
cream base cream
Medical Disclaimer