AK-POLY-BAC: Package Insert / Prescribing Info
Package insert / product label
Generic name: bacitracin zinc and polymyxin b sulfate
Dosage form: ophthalmic ointment
Drug class: Ophthalmic anti-infectives
Medically reviewed by Drugs.com. Last updated on Jan 13, 2025.
On This Page
AK-POLY-BAC Description
Bacitracin Zinc and Polymyxin B Sulfate Ophthalmic Ointment is a sterile antimicrobial ointment for ophthalmic use.
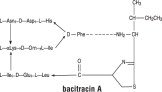
Bacitracin zinc is the zinc salt of bacitracin, a mixture of related cyclic polypeptides (mainly bacitracin A) produced by the growth of an organism of the licheniformis group of Bacillus subtilis var Tracy. It has a potency of not less than 40 bacitracin units per mg. The structural formula for bacitracin A is:
Polymyxin B Sulfate is the sulfate salt of polymyxin B1 and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on an anhydrous basis. The structural formulae are:
Each gram contains: Bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units, white petrolatum and mineral oil.
AK-POLY-BAC - Clinical Pharmacology
Polymyxin B attacks gram-negative bacilli, including virtually all strains of Pseudomonas aeruginosa and H influenzae species.
Bacitracin is active against most gram-positive bacilli and cocci including hemolytic streptococci.
Indications and Usage for AK-POLY-BAC
For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to bacitracin zinc and polymyxin B sulfate.
Contraindications
This product is contraindicated in those individuals who have shown hypersensitivity to any of its components.
Related/similar drugs
Precautions
As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. Appropriate measures should be taken if this occurs.
AK-POLY-BAC Dosage and Administration
Apply the ointment every 3 or 4 hours for 7 to 10 days, depending on the severity of the infection.
How is AK-POLY-BAC supplied
Bacitracin Zinc and Polymyxin B Sulfate ophthalmic ointment USP, sterile, each gram contains bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units, in a tube of 3.5 g (1/8 oz) with ophthalmic tip.
NDC 17478-238-35
Storage and Handling
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Akorn
Manufactured By: Akorn, Inc.
Lake Forest, IL 60045
AKP00N
Rev. 06/16
| AK-POLY-BAC
bacitracin zinc and polymyxin b sulfate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akorn (117696770) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696840 | MANUFACTURE(17478-238) , ANALYSIS(17478-238) , STERILIZE(17478-238) , PACK(17478-238) , LABEL(17478-238) | |
Frequently asked questions
More about AK-Poly-Bac (bacitracin / polymyxin b ophthalmic)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: ophthalmic anti-infectives