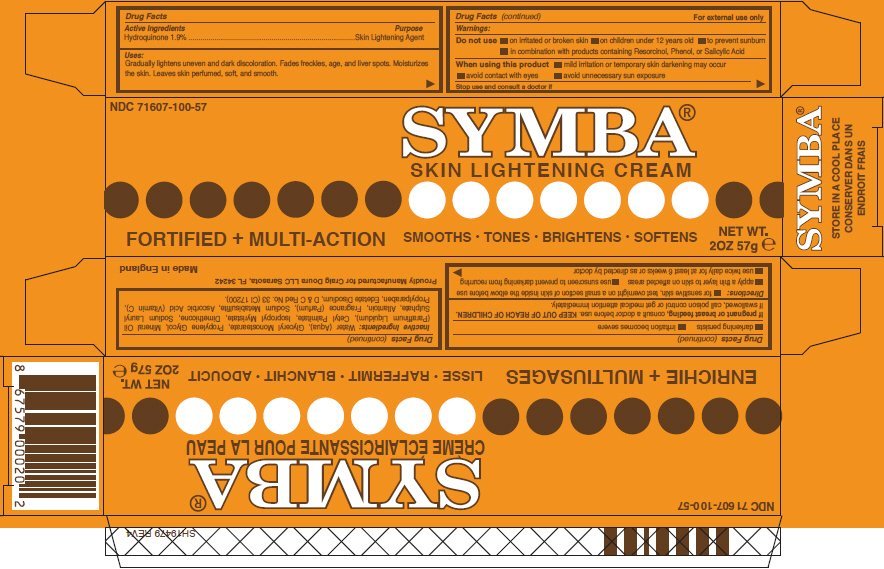
SYMBA Skin Lightening
Dosage form: cream
Ingredients: Hydroquinone 19mg in 1g
Labeler: Craig Doura LLC
NDC code: 71607-100
Medically reviewed by Drugs.com. Last updated on Sep 25, 2023.
Drug Facts
Hydroquinone 1.9%
Skin Lightening Agent
Gradually lightens uneven and dark discoloration. Fades freckles, age, and liver spots. Moisturizes the skin. Leaves skin perfumed, soft, and smooth.
For external use only
- on irritated or broken skin
- on children under 12 years old
- to prevent sunburn
- in combination with products containing Resorcinol, Phenol, or Salicylic Acid
- mild irritation or temporary skin darkening may occur
- avoid contact with eyes
- avoid unnecessary sun exposure
- darkening persists
- irritation becomes severe
If pregnant or breast feeding, consult a doctor before use.
KEEP OUT OF REACH OF CHILDREN. If swallowed, call poison control or get medical attention immediately.
- for sensitive skin, test overnight on a small section of skin inside the elbow before use
- apply a thin layer to skin on affected areas
- use sunscreen to prevent darkening from recurring
- use twice daily for at least 6 weeks or as directed by doctor
Water (Aqua), Glyceryl Monostearate, Propylene Glycol, Mineral Oil (Paraffinum Liquidum), Cetyl Palmitate, Isopropyl Myristate, Dimethicone, Sodium Lauryl Sulphate, Allantoin, Fragrance (Parfum), Sodium Metabisulfite, Ascorbic Acid (Vitamin C), Propylparaben, Edetate Disodium, D & C Red No. 33 (CI 17200).
| SYMBA SKIN LIGHTENING
hydroquinone cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Craig Doura LLC (079084370) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.