GDC 119 Pill: white, round
The pill with imprint GDC 119 (White, Round, 0mm) has been identified as Genaton aluminum hydroxide 80 mg / magnesium trisilicate 20 mg.
Images for GDC 119
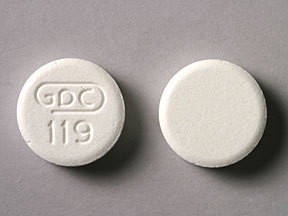
Genaton
- Imprint
- GDC 119
- Strength
- aluminum hydroxide 80 mg / magnesium trisilicate 20 mg
- Color
- White
- Shape
- Round
- Labeler / Supplier
- Teva Pharmaceuticals USA
- National Drug Code (NDC)
- 00182-2220 (Discontinued)
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
