FDA Targets Drug Side Effects
On This Page:
The Food and Drug Administration (FDA) is working on many fronts to expect the unexpected when it comes to medication side effects.
FDA wants to strengthen researchers’ ability to detect chemical or biological substances that could be life-threatening, or even fatal, before products containing them hit the market.
“All drugs have side effects,” says FDA’s Vicki Seyfert-Margolis, Ph.D., senior advisor for science innovation and policy. The goal is to “predict side effects earlier and better” to help ensure that patients avoid them altogether.
COX-2 inhibitors—anti-inflammatory drugs designed to ease arthritis pain while protecting the stomach and intestines—are a good example of popular medicines that had unforeseen complications, says Douglas Throckmorton, M.D., deputy director of FDA’s Center for Drug Evaluation and Research.
These drugs were found to increase the risk of heart attack and stroke. The drugs Vioxx and Bextra were withdrawn from the market in 2004 and 2005 respectively. FDA concluded that the benefits of Celebrex, another COX-2 inhibitor, outweigh the potential risks in properly selected and informed patients.
Agency officials say that some side effects only come to light when a medication is used by a larger and more diverse group of people than the one involved in the research prior to approval. For regulators, this raises the question: Are there better ways that we can be testing these drugs to more accurately predict these important health risks?
That question is at the foundation of a kind of scientific research called predictive toxicology. Toxicology is the study of chemical or biological substances that can be harmful to people, plants and animals.
Predictive toxicology is getting “the best possible picture” of the risks that would be involved in taking a drug before it is even tested in human patients, says Suzanne Fitzpatrick, Ph.D., senior science advisor at FDA.
In August 2011, FDA released its “Strategic Plan for Regulatory Science,” an initiative that identified modernizing the agency’s work in the field of toxicology as a priority. Regulatory science is the science of developing new tools, standards, and approaches to assess the safety, effectiveness, quality and performance of FDA-regulated products.
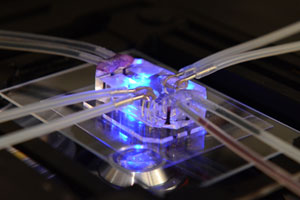
‘Human on a Chip’
FDA is working with the federal Defense Advanced Research Projects Agency to create the “human on a chip,” a technology that could revolutionize drug testing by using human cells to create a miniature version of 10 different organ systems that mirror their function in a full-size human body.
Fitzpatrick says this technology will allow researchers to test new drugs safely and more accurately without having to use animals as test subjects.
Working in the same vein to improve drug testing, researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University have developed a “Lung-on-a-Chip.” They used lung tissue, cells and other materials to mimic the physical and chemical behaviors of a living, breathing human lung.
These technologies are still works in progress but, Fitzpatrick says, “It’s what the future might look like.”
A Strategic Plan
FDA’s National Center for Toxicological Research (NCTR) in Jefferson, Ark., investigates the toxicity of many products, including medications.
NCTR Center Director William Slikker Jr., Ph.D., says that the testing process is often complicated by the fact that many of these tests are not usually conducted on the “target species”—the people who will eventually be using these products.
FDA is working towards refining, reducing or replacing tests that involve animals, most often rodents, with other technologies. Slikker says animals can be problematic because it is difficult to simulate certain conditions that plague the human body.
Slikker continued, “It is clear that traditional testing methods for identifying hazards and finding the right doses need to be improved. It happens too often that drugs pass initial tests, only to fail later.”
NCTR is working on developing and perfecting tests that will more accurately anticipate how the human body will react to new drugs, materials, and other products. They include:
- The “omics”—This area of science, which includes genomics, proteomics and metabolomics, has many functions involving how genes, proteins and molecules interact with medical products in the body. Slikker says these advanced tools are used to chart how genes and proteins react when they are exposed to certain chemicals.
- Bioimaging—This noninvasive tool can be particularly useful in animal studies because it allows researchers to collect data repeatedly throughout the life span of an animal. These technologies include magnetic resonance imaging (MRI), which uses a magnetic field and radio wave energy to create images of the inside of the body.
- Mathematical modeling—Once the body’s biological processes are understood, scientists can create mathematical models that use math formulas or steps to predict how long a drug will remain in the body and to help define a drug’s interactions in a human body.
- In vitro testing—This work is conducted in a laboratory using cells and tissues of human or animal origin. The test systems are exposed to certain ingredients that would be in a particular drug to determine at what point the cells become unhealthy, if at all.
Slikker says there is much work to be done. Scientific understanding of human health and individual genetic makeup may lead to the rapid creation of new targets for drug development. And FDA’s ability to develop equally innovative methods to test those targets and support efficient product development must keep pace with these advances, he says.
September 17, 2012
Return to FDA Consumer Articles