M MT1 Pill - white round, 7mm
Pill with imprint M MT1 is White, Round and has been identified as Metoprolol Succinate Extended-Release 25 mg. It is supplied by Mylan Pharmaceuticals Inc.
Metoprolol is used in the treatment of Angina; High Blood Pressure; Heart Failure; Angina Pectoris Prophylaxis; Heart Attack and belongs to the drug class cardioselective beta blockers. Risk cannot be ruled out during pregnancy. Metoprolol 25 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for M MT1
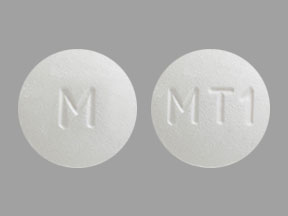
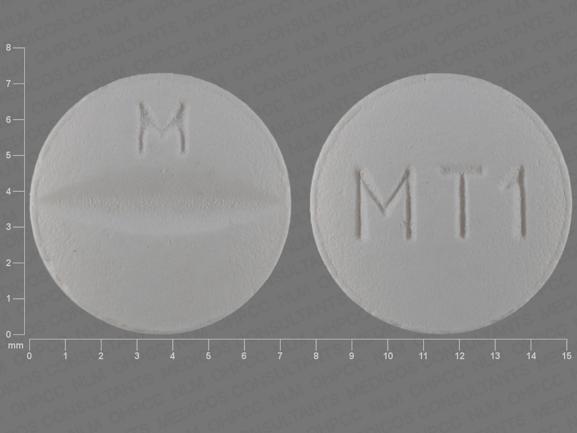
Metoprolol Succinate Extended-Release
- Imprint
- M MT1
- Strength
- 25 mg
- Color
- White
- Size
- 7.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Cardioselective beta blockers
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Mylan Pharmaceuticals Inc.
- National Drug Code (NDC)
- 00378-4595
- Inactive Ingredients
-
silicon dioxide,
ethylcellulose,
hypromelloses,
lactose monohydrate,
microcrystalline cellulose,
polyethylene glycol,
sodium stearyl fumarate,
titanium dioxide,
triacetin
Note: Inactive ingredients may vary.
Related images for "M MT1"
More about metoprolol
- Check interactions
- Compare alternatives
- Reviews (646)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: cardioselective beta blockers
- Breastfeeding
- En español
Patient resources
- Metoprolol drug information
- Metoprolol (Intravenous) (Advanced Reading)
- Metoprolol Extended-Release Tablets
- Metoprolol Tablets
- Metoprolol Extended-Release Capsules
- Metoprolol Injection
Other brands
Professional resources
- Metoprolol monograph
- Metoprolol (FDA)
- Metoprolol Succinate (FDA)
- Metoprolol Succinate ER Capsules (FDA)
- Metoprolol Succinate ER Tablets (FDA)
Other brands
Toprol-XL, Lopressor, Kapspargo Sprinkle
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.