Duetact Dosage
Generic name: pioglitazone hydrochloride 30mg, glimepiride 2mg
Dosage form: tablet
Drug class: Antidiabetic combinations
Medically reviewed by Drugs.com. Last updated on Oct 24, 2022.
Recommendations for All Patients
DUETACT should be taken once daily with the first main meal.
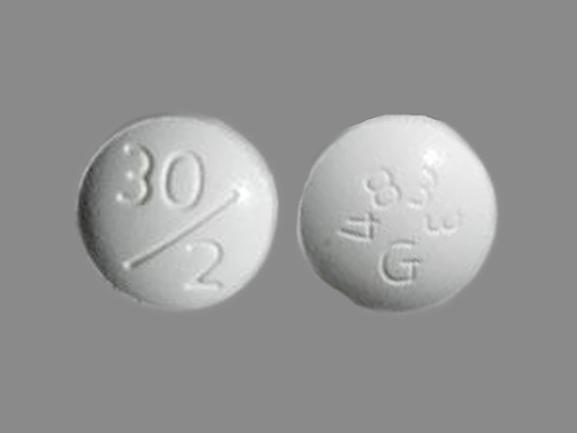
DUETACT tablets are available as a 30 mg pioglitazone plus 2 mg glimepiride or a 30 mg pioglitazone plus 4 mg glimepiride tablet. If therapy with a combination tablet containing pioglitazone and glimepiride is considered appropriate the recommended starting dose is:
- •
- 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- •
- for patients inadequately controlled on glimepiride monotherapy: 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- •
- for patients inadequately controlled on pioglitazone monotherapy: 30 mg/2 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- •
- for patients who are changing from combination therapy of pioglitazone plus glimepiride as separate tablets: DUETACT should be taken at doses that are as close as possible to the dose of pioglitazone and glimepiride already being taken,
- •
- for patients currently on a different sulfonylurea monotherapy or switching from combination therapy of pioglitazone plus a different sulfonylurea (e.g., glyburide, glipizide, chlorpropamide, tolbutamide, acetohexamide): 30 mg/2 mg once daily and adjusted after assessing adequacy of therapeutic response. Observe for hypoglycemia for one to two weeks due to the potential overlapping drug effect.
- •
- for patients with systolic dysfunction, the lowest approved dose of DUETACT should be prescribed only after titration from 15 mg to 30 mg of pioglitazone has been safely tolerated.
After initiation of DUETACT or with dose increase, monitor patients carefully for hypoglycemia and adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure [see Boxed Warning and Warnings and Precautions (5.7)].
Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating DUETACT. Routine periodic monitoring of liver tests during treatment with DUETACT is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of DUETACT or who are found to have abnormal liver tests while taking DUETACT should be managed as described under Warnings and Precautions [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
Concomitant Use with an Insulin Secretagogue or Insulin
If hypoglycemia occurs in a patient coadministered DUETACT and an insulin secretagogue, the dose of the insulin secretagogue should be reduced.
If hypoglycemia occurs in a patient coadministered DUETACT and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
Concomitant Use with Strong CYP2C8 Inhibitors
Coadministration of pioglitazone and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors. If gemfibrozil or other CYP2C8 inhibitors need to co-administered, patients should switch to individual components of DUETACT because the minimum dose of pioglitazone in DUETACT exceeds 15 mg [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Concomitant Use with Colesevelam
When colesevelam is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, DUETACT should be administered at least four hours prior to colesevelam [see Drug Interactions (7.7) and Clinical Pharmacology (12.3)].
More about Duetact (glimepiride / pioglitazone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- Generic availability
- FDA approval history
- Drug class: antidiabetic combinations
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.