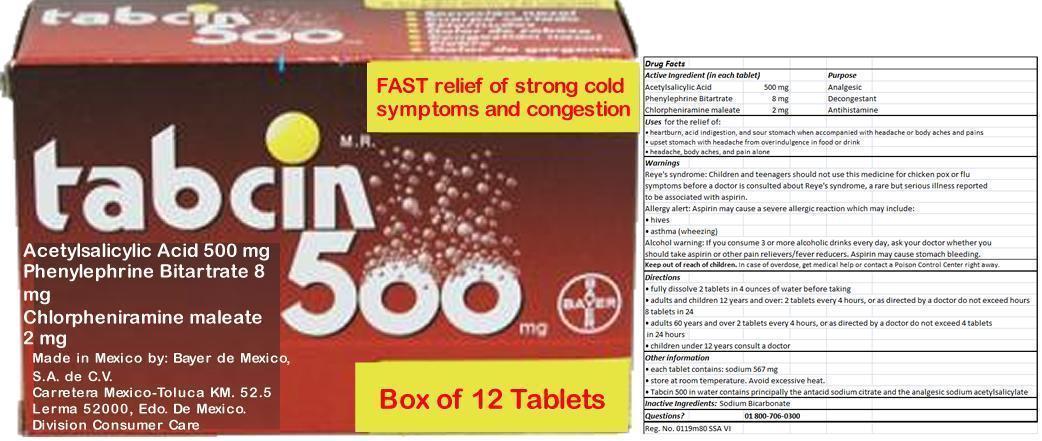
TABCIN 500
Dosage form: tablet, effervescent
Ingredients: ASPIRIN 500mg, Phenylephrine Bitartrate 8mg, Chlorpheniramine maleate 2mg
Labeler: Salimex, S.A.
NDC code: 53666-408
Medically reviewed by Drugs.com. Last updated on Sep 2, 2024.
(in each tablet)
Acetylsalicylic Acid 500 mg
Phenylephrine Bitartrate 8 mg
Chlorpheniramine maleate 2 mg
Acetylsalicylic Acid 500 mg Analgesic
Phenylephrine Bitartrate 8 mg Decongestant
Chlorpheniramine maleate 2 mg Antihistamine
In case of overdose, get medical help or contact a Poison Control Center right away.
- heartburn, acid indigestion, and sour stomach when accompanied with headache or body aches and pains
- upset stomach with headache from overindulgence in food or drink
- headache, body aches, and pain alone
Reye's syndrome: Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye's syndrome, a rare but serious illness reported to be associated with aspirin.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- asthma (wheezing)
Alcohol warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
- fully dissolve 2 tablets in 4 ounces of water before taking
- adults and children 12 years and over: 2 tablets every 4 hours, or as directed by a doctor do not exceed 8 tablets in 24 hours
- adults 60 years and over 2 tablets every 4 hours, or as directed by a doctor do not exceed 4 tablets in 24 hours
- children under 12 years consult a doctor
Other information
- each tablet contains: sodium 567 mg
- store at room temperature. Avoid excessive heat.
- Tabcin 500 in water contains principally the antacid sodium citrate and the analgesic sodium acetylsalicylate
Sodium Bicarbonate
01 800-706-0300
| TABCIN
500
acetylsalicylic acid, phenylephrine bitartrate, chlorpheniramine maleate tablet, effervescent |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Salimex, S.A. (589201581) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bayer de Mexico, S.A. de CV | 588165709 | manufacture(53666-408) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.