GYNO-BETADINE
Dosage form: suppository
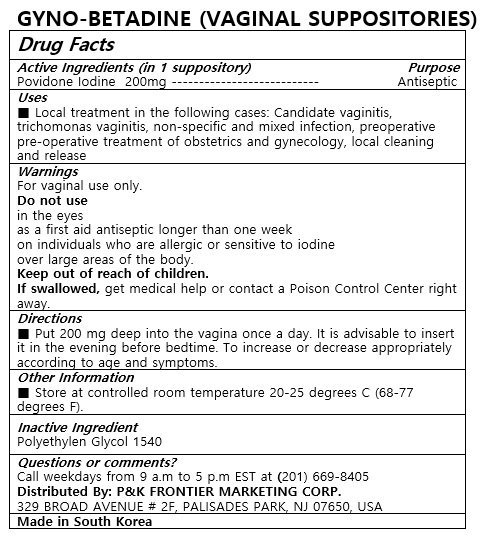
Ingredients: POVIDONE-IODINE 200mg
Labeler: OASIS TRADING
NDC code: 72689-0042
Medically reviewed by Drugs.com. Last updated on Mar 11, 2024.
POVIDONE-IODINE
Local treatment in the following cases: Candidate vaginitis, trichomonas vaginitis, non-specific and mixed infection, preoperative pre-operative treatment of obstetrics and gynecology, local cleaning and release
Keep out of reach of children
Put 200 mg deep into the vagina once a day. It is advisable to insert it in the evening before bedtime. To increase or decrease appropriately according to age and symptoms.
Do not use
in the eyes
as a first aid antiseptic longer than one week
on individuals who are allergic or sensitive to iodine
over large areas of the body
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
POLYETHYLENE GLYCOL 1540
For vaginal use only
| GYNO-BETADINE
povidone iodine suppository |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - OASIS TRADING (689991468) |
| Registrant - OASIS TRADING (689991468) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OASIS TRADING | 689991468 | manufacture(72689-0042), relabel(72689-0042) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.