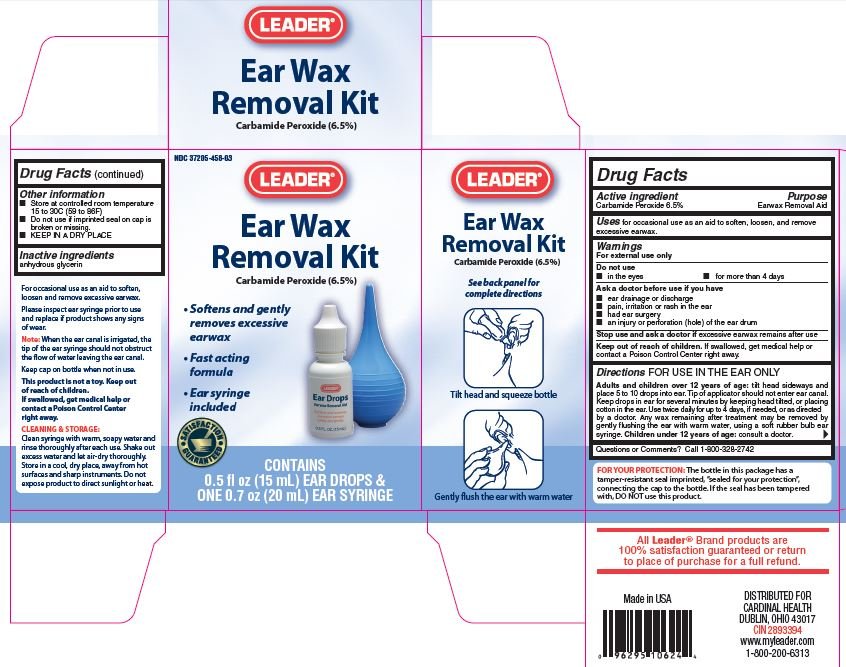
Earwax Removal Kit
Dosage form: liquid
Ingredients: CARBAMIDE PEROXIDE 6.5mg in 100mL
Labeler: Cardinal Health
NDC code: 37205-458
Medically reviewed by Drugs.com. Last updated on Nov 13, 2023.
Carbamide Peroxide 6.5%
Earwax Removal Aid
For occasional use to soften, loosen and remove excessive earwax.
For External Use Only
- In the eye
- for more than 4 days
- ear drainage or discharge
- pain, irritation or rash in the ear
- had ear surgery
- an injury or perforation (hole) in the ear drum
Stop use and ask a doctor if excessive earwax remains after use
If swallowed, get medical help or contact a Poison Control Center right away.
FOR USE IN THE EAR ONLY
Adults and children 12 years of age: tilt head sideways and place 5 to 10 drops into ear. Tip of applicator should not enter ear canal. Keep drops in ear for several minutes by keeping head tilted, or placing cotton in the ear. Use twice daily for up to 4 days, if needed, or as directed by a doctor. Any wax remaining after treatment my be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe. Children under 12 years of age, consult a doctor.
Do not use if imprinted seal on cap is broken or missing.
- Store at controlled room temperature 15 to 30C (59 to 86F)
- KEEP IN A DRY PLACE
anhydrous Glycerin
Call 1-800-328-2742
| EARWAX REMOVAL KIT
earwax removal kit liquid |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Cardinal Health (097537435) |
| Registrant - Continental Manufacturing Chemist, Inc. (005278007) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.