The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
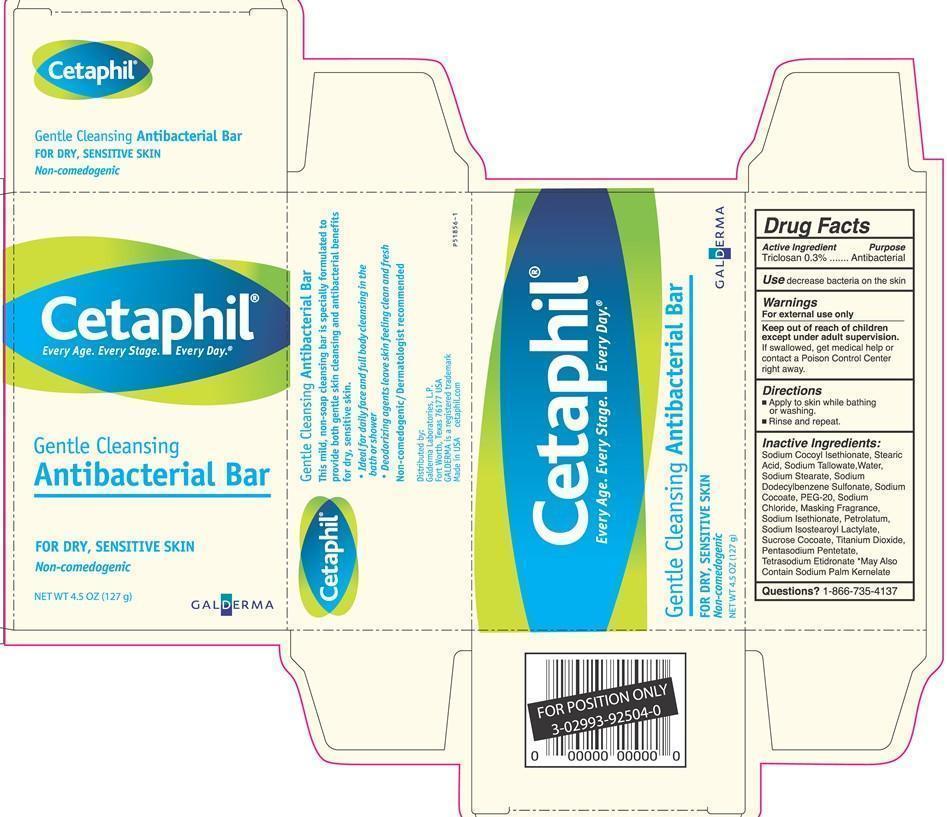
Cetaphil Gentle Cleansing Antibacterial Bar
Dosage form: soap
Ingredients: TRICLOSAN 0.3g in 100g
Labeler: Galderma Laboratories, L.P.
NDC code: 0299-3925
Triclosan 0.3%
Antibacterial
decrease bacteria on the skin
For external use only
If swallowed, get medical help or contact a Poison Control Center right away.
- Apply to skin while bathing or washing.
- Rinse and repeat.
Sodium Cocoyl Isethionate, Stearic Acid, Sodium Tallowate, Water, Sodium Stearate, Sodium Dodecylbenzene Sulfonate, Sodium Cocoate, PEG-20, Sodium Chloride, Masking Fragrance, Sodium Isethionate, Petrolatum, Sodium Isostearoyl Lactylate, Sucrose Cocoate, Titanium Dioxide, Pentasodium Pentetate, Tetrasodium Etidronate *May Also Contain Sodium Palm Kernelate
1-866-735-4137
Cetaphil®
Gentle Cleansing Antibacterial Bar
FOR DRY, SENSITIVE SKIN
Non-comedogenic
Cetaphil®
Every Age. Every Stage. Every Day.
Gentle Cleansing
Antibacterial Bar
FOR DRY, SENSITIVE SKIN
Non-comedogenic
NET WT 4.5 oz (127 g)
GALDERMA
Cetaphil®
Gentle Cleansing Antibacterial Bar
This mild, non-soap cleansing bar is specially formulated to
provide both gentle skin cleansing and antibacterial benefits for dry, sensitive skin.
- Ideal for daily face and full body cleansing in the bath or shower
- Deodorizing agents leave skin feeling clean and fresh
Non-comedogenic/Dermatologist recommended
Marketed by:
Galderma Laboratories, LP.
Fort Worth, Texas 76177 USA
GALDERMA is a registered trademark
Made in USA
cetaphil.com
P51856-1
Cetaphil®
Every Age. Every Stage. Every Day.
Gentle Cleansing Antibacterial Bar
FOR DRY, SENSITIVE SKIN
Non-comedogenic
NET WT 4.5 oz (127 g) GALDERMA
| CETAPHIL GENTLE CLEANSING ANTIBACTERIAL BAR
triclosan soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bradford Soap Works, Inc. | 001201045 | manufacture(0299-3925) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.