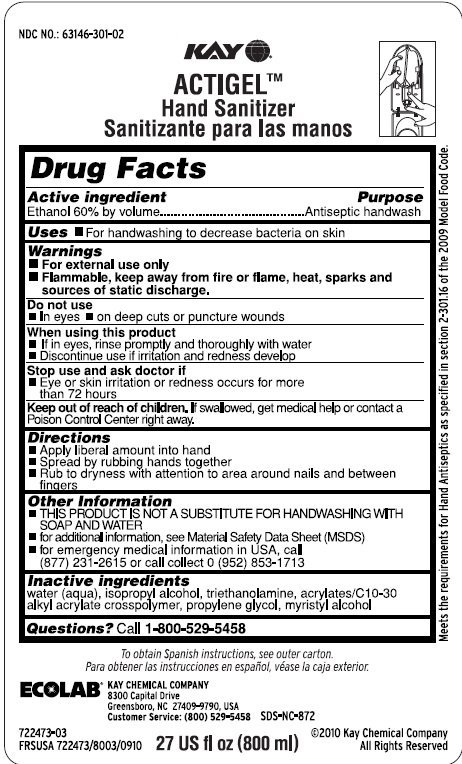
Kay Actigel
Dosage form: solution
Ingredients: ALCOHOL 60mL in 100mL
Labeler: Kay Chemical Company
NDC code: 63146-301
Medically reviewed by Drugs.com. Last updated on Feb 16, 2024.
Ethanol 60% by volume
Antiseptic handwash
- For handwashing to decrease bacteria on the skin
- For external use only
- Flammale, keep away from fire or flame, heat, sparks and sources of static discharge.
- In eyes
- On deep cuts or puncture wounds
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
- Eye or skin irritation and redness persist for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Apply liberal amount into hand
- Spread by rubbing hands together
- Rub to dryness with attention to area around nails and between fingers
- THIS PRODUCT IS NOT A SUBSTITUTE FOR HANDWASHING WITH SOAP AND WATER
- For additional information, see Material Safety Data Sheet (MSDS)
- For emergency medical information in the USA call 1 (877) 231-2615 or call collect 0 (952)853-1713
water (aqua), isopropyl alcohol, triethanolamine, acrylates/C10-C30 alkyl acrylate crosspolymer, propylene glycol, myristyl alcohol
Questions? call 1-800-529-5458
NDC NO.: 63146-301-02
Kay
Actigel
Hand Sanitizer
To obtain instruction in Spanish, see outer carton.
ECOLAB
KAY CHEMICAL COMPANY
8300 Capital Drive
Greensboro, NC 27409-9790, USA
Customer Service: (800) 529-5458
SDS-NC-872
27 US fl oz (800 ml)
722473-03
FRSUSA 722473/8003/0910
©2010 Kay Chemical Company
All Rights Reserved
| KAY ACTIGEL
ethanol solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Kay Chemical Company (003237021) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.