Ropivacaine FDA Alerts
The FDA Alerts below may be specifically about ropivacaine or relate to a group or class of drugs which include ropivacaine.
MedWatch Safety Alerts are distributed by the FDA and published by Drugs.com. Following is a list of possible medication recalls, market withdrawals, alerts and warnings.
Recent FDA Alerts for ropivacaine
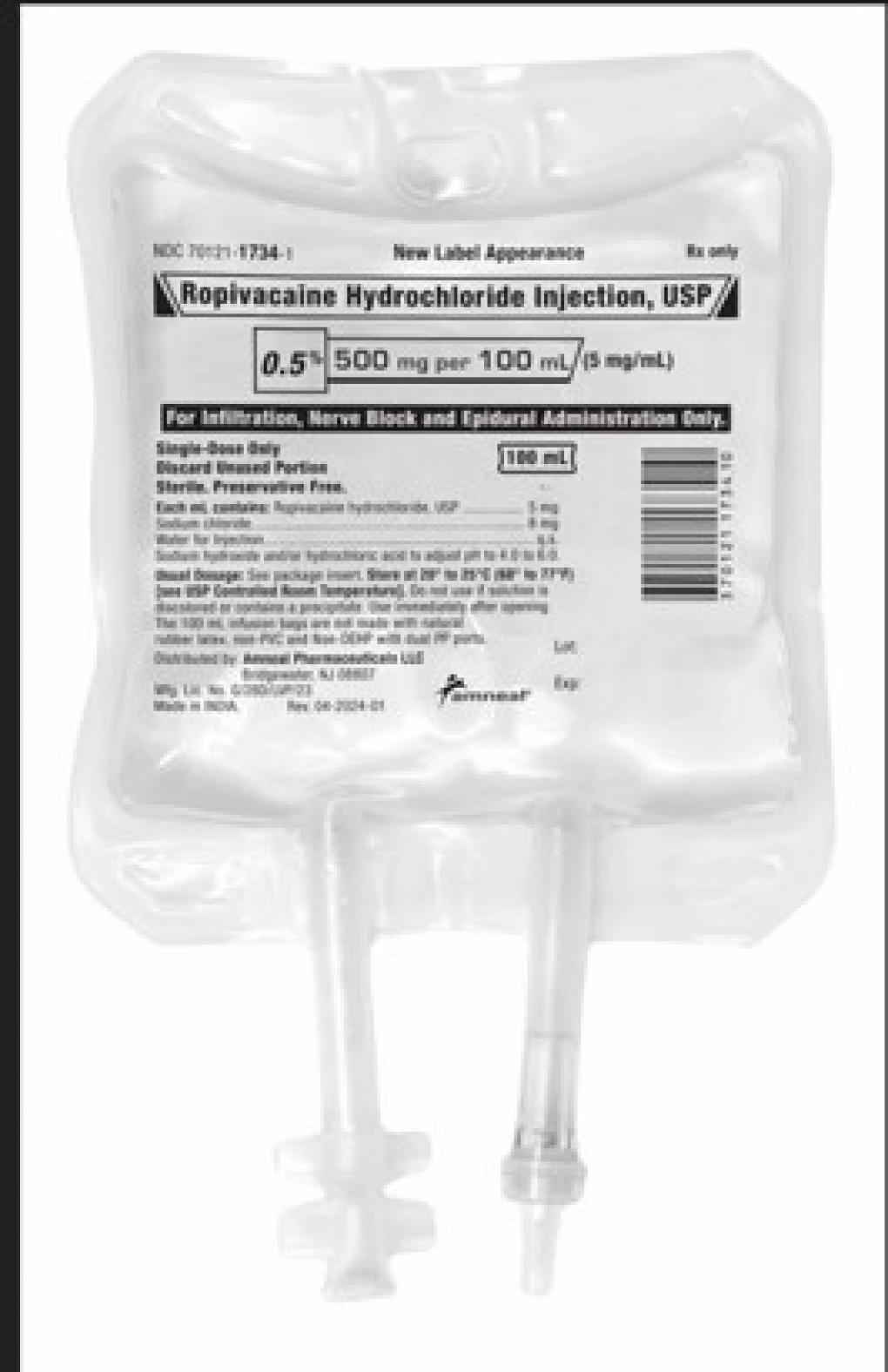
Amneal Pharmaceutical LLC Issues a Nationwide Recall of Ropivacaine Hydrochloride Injection, USP 500mg/100mL, Due to the Potential Presence of Particulate Matter
April 18, 2025 -- Bridgewater, NJ, Amneal Pharmaceutical LLC, is recalling two lots of Ropivacaine Hydrochloride Injection, USP, 500mg/100mL, Infusion bags to the hospital/user level as the products may contain an inert fiber identified as polypropylene fibers from the IV bag.
Risk Statement: Introduction of polypropylene particulates into the epidural space (or inadvertent administration into the intrathecal space) may result in a variety of adverse events. There is a reasonable probability that particulate matter in the epidural space may cause an epidural inflammatory process to meningitis or potentially damage the spinal cord. Administered intrathecally, particulate matter could result in inflammation, hydrocephalus (water on the brain), which could lead to embolization and organ damage.
To date, Amneal Pharmaceuticals has received no reports of adverse events or injuries related to this recall.
The recalled product was distributed nationwide to wholesalers/distributors between the dates of 04/23/2024 to 11/8/2024 only.
The product is indicated for the production of local or regional anesthesia for surgery and or acute pain management and is packaged in 12x100mL Single Dose IV bags (NDC 70121-17343). The affected Ropivacaine Hydrochloride Injection, USP, 500mg/100mL, products are Lot AL240003 (exp 01/2026) and Lot AL240004 (exp 01/2026). No other Ropivacaine Hydrochloride Injection, USP lots are impacted.
Amneal is notifying its customers by UPS and is arranging for return of all recalled products. Wholesalers/distributors are asked to notify their hospital/ user customers of the recall and provide instruction to contact Amneal for the return of the recalled products to Amneal.
Hospitals/users with questions regarding this recall can contact Amneal Pharmaceuticals by:
Phone: 833-582-0812 Monday-Friday, 8:00 am-5:00 pm, EST
Fax: 631-983-2595
E-mail to: RopivacaineHCl-Recall@amneal.com
For Medical Inquiries or to report Adverse Events, or quality problems experienced with the use of this product, please contact Amneal Drug Safety by phone at 1-877-835-5472, Monday - Friday, 8:00 am – 6:00 pm, EST, or e-mail at DrugSafety@amneal.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration
Product Photos

Source: FDA
Local Anesthetics, Continuously Infused (marketed as bupivacaine, chloroprocaine, lidocaine, mepivacaine, procaine, ropivacaine) - Chondrolysis
FDA notified healthcare professionals of 35 reports of chondrolysis (necrosis and destruction of cartilage) in patients given continuous intra-articular infusions of local anesthetics (marketed as bupivacaine, chloroprocaine, lidocaine, mepivacaine, procaine, ropivacaine) with elastomeric infusion devices to control post-surgical pain. The local anesthetics (with and without epinephrine) were infused for extended periods of time (48 to 72 hours) directly into the intra-articular space using an elastomeric pump. Joint pain, stiffness, and loss of motion were reported as early as the second month after receiving the infusion. In more than half of these reports, the patients required additional surgery, including arthroscopy or arthroplasty (joint replacement).
Local anesthetics are approved as injections for the production of local or regional anesthesia or analgesia. The approved drug labels for local anesthetics do not include an indication for continuous intra-articular postoperative infusions or use of infusion devices, such as elastomeric pumps. The FDA has not cleared any infusion devices with an indication for use in intra-articular infusion of local anesthetics. Health care professionals are encouraged to follow the instructions for use of elastomeric infusion devices, and to not use these devices for continuous intra-articular infusion of local anesthetics after orthopedic surgery.
This notice provides further management considerations for healthcare professionals, information for patients, a data summary of the 35 reports, and references.
[11/13/2009 - Information for Healthcare Professionals - FDA]
