The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Slentrol
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
(dirlotapide)
Oral solution for use in dogs only.
Slentrol Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
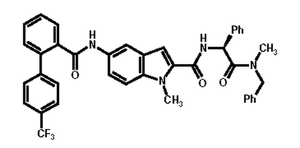
SLENTROL (dirlotapide) is a solution formulated at a concentration of 5 mg/mL of dirlotapide for oral administration to dogs. Dirlotapide is a selective microsomal triglyceride transfer protein inhibitor that blocks the assembly and release of lipoprotein particles into the bloodstream (via the lymphatic system) in dogs.1 The empirical formula is C40H33F3N4O3 and the molecular weight is 674.73. The chemical name is (S) - N - {2 - [Benzyl(methyl)amino] - 2 - oxo - 1 - phenylethyl} - 1 - methyl - 5 - [4’ - (trifluoromethyl)[1,1’ - biphenyl] - 2 - carboxamido] - 1H - indole - 2 - carboxamide.
The chemical structure of dirlotapide is:
Slentrol Indications
SLENTROL (dirlotapide) Oral Solution is indicated for the management of obesity in dogs.
Dosage and Administration
SLENTROL should be prescribed as part of an overall weight management program that incorporates a complete and balanced canine diet and physical activity.Dietary Considerations: SLENTROL should only be used with a commercial maintenance diet. Any diet changes should be made gradually and prior to initiating SLENTROL treatment. Better appetite control may be achieved in diets that are not restricted in fat content. Changing the diet while initiating treatment with SLENTROL should be avoided.
The dog will need to be weighed at the start of treatment and then at monthly intervals so that the dosing regimen can be adjusted according to the prescribing instructions below.
During the first month of therapy, the dosing regimen for SLENTROL consists of two fixed dose rates (number of mL administered per unit of body weight) in all dogs. In subsequent months of therapy, the recommended dosing regimen prescribed for SLENTROL varies for each individual dog and the dose volume must be specifically calculated each month, based on the amount of weight lost (expressed as a percent) during the previous month of therapy.
With regard to dosing it is important to note that:
- Initial body weight is used to calculate the dose that is first administered.
- Subsequent dose adjustments are made by adjusting the volume of solution administered.
- Dose adjustments are determined at monthly intervals.
- A diet change at the time of a dose escalation should be avoided.
The dose should not exceed a maximum daily dose of 0.2 mL/kg (0.09 mL/lb), based on the dog’s current body weight, during any part of treatment.
Dose Preparation and Administration: To prepare for oral administration, remove the bottle cap and insert the supplied oral dosing syringe through the membrane into the bottle. Invert the bottle and withdraw the appropriate volume required using the graduation marks on the side of the oral dosing syringe.
The 1 mL dosing syringe provided with SLENTROL can not accurately measure the dosage required to treat dogs that weigh less than 12.5 lb (5.7 kg).
A veterinarian or veterinary technician should instruct the pet owner/caregiver on how to measure the amount of SLENTROL to be administered to ensure accurate dosing.
SLENTROL can be administered directly into the dog’s mouth or on a small amount of food. It can be given with a meal or at a different time of day.
Wipe the oral dosing syringe clean after each use with a clean dry cloth or disposable towel. Do not introduce water into the oral dosing syringe or the SLENTROL solution.
Weight Loss Phase
Initial assessment and dosing in first month
Assess the dog prior to initiation of therapy with SLENTROL to determine the desired weight and to assess the animal’s general health (See Precautions).
The initial dosage of SLENTROL is 0.01 mL/kg (0.0045 mL/lb) body weight, administered once daily, orally, for the first 14 days. After the first 14 days of treatment, the dose volume of SLENTROL should be doubled to 0.02 mL/kg (0.009 mL/lb) of body weight, administered once daily for the next 14 days (days 15 to 28 of treatment).
Subsequent Monthly Dose Adjustments for Weight Loss
Dogs should be weighed monthly and the dose volume adjusted every month, as necessary, to maintain a target percent weight loss of ≥0.7% per week.
If the dog has gained weight since the last visit, the dose volume should be increased. Go directly to the First (or Subsequent) Dose Adjustment Section below.
If the dog has lost weight, determine if an adjustment in dose is required using the following calculations:
(Number of weeks between visits) X 0.7 % per week = Target % weight loss
Example - in 4 weeks (28 days) the Target weight loss would be 4 X 0.7% per week, or at least 2.8% of the total body weight
(Weight at last visit - Weight at current visit / Weight at last visit) X 100 = Actual % loss
Compare the Target % weight loss (of ≥0.7% per week) with the Actual % weight loss for that dog.
To determine if a dose adjustment is necessary, compare the Actual % weight loss to the Target % weight loss and use the following guidelines. Note: All dose adjustments are based solely on volume (mL).
Monthly Weight Loss Rate Achieved
- If the Actual % weight loss is the same or greater than the Target % weight loss, the dose volume (number of mL administered each day) should remain the same for the next month of dosing until the next scheduled assessment.
Monthly Weight Loss Not Achieved
- If the Actual % weekly weight loss is less than the Target % weight loss of 0.7% weekly, the following dose adjustment instructions apply:
- First Dose Adjustment
- The dose volume (number of mL administered each day) should be increased by 100%, resulting in an increase of the dose volume to 2.0 times the dose administered during the previous month of dosing. Only perform a 100% dose increase once during treatment after day 14.
- Subsequent Dose Adjustments
- If additional dose increases are necessary in the following months, the dose volume (number of mL administered each day) should be increased by 50%, resulting in an increase of the dose volume to 1.5 times the dose administered the previous month of dosing. Based on the dog’s current body weight a daily dose of 0.2 mL/kg (0.09 mL/lb) should not be exceeded.
If a dog’s food consumption is greatly reduced for several consecutive days, the dose may be withdrawn until the appetite returns (usually 1-2 days) and then resume dosing at the same volume.
The monthly adjustments should continue in this way until the desired weight determined at the start of therapy is reached. When the desired weight is reached, begin the weight management phase.
Weight Management Phase
A 3-month weight management phase is recommended to successfully maintain the weight loss achieved with treatment. During the weight management phase, the veterinarian and the pet owner should establish the optimal level of food intake and physical activity needed. SLENTROL administration should be continued during the weight management phase until the dog owner can establish the food intake and physical activity needed to stabilize body weight at the dog’s desired weight.
To dose for weight management, body weight should continue to be assessed at monthly intervals.
- First Dose Adjustment
- If the dog lost ≥1% body weight per week in the last month of the weight loss phase, the dose volume (number of mL administered each day) should be decreased by 50% resulting in a decrease of the dose volume to 0.5 times the dose administered the previous month.
- If the dog lost between 0 and 1% the dose should remain the same.
- If the dog gained weight, the dose should be increased by 50% resulting in an increase of the dose volume to 1.5 times the dose administered the previous month.
- Subsequent Dose Adjustments
In subsequent months the dose volume should be increased or decreased by 25% to maintain a constant weight.
- If the dog is within -5% to +5% of the body weight at the end of the weight loss phase, the dose volume (number of mL administered each day) should remain unchanged.
- If the dog lost >5% body weight, then the dose should be decreased by 25%.
- If the dog gained > 5% body weight, then the dose should be increased by 25%. Based on the dog’s current body weight a daily dose of 0.2 mL/kg (0.09 mL/lb) should not be exceeded.
When SLENTROL is discontinued, the daily amount of food offered and physical activity should be continued as established during the weight management phase. Reverting to previous food intake or physical activity levels at this point can contribute to a re-gain of some or all of the weight loss that has been achieved.
The safety of SLENTROL use in dogs has not been evaluated beyond 1 year.
INFORMATION FOR OWNER OR PERSON TREATING ANIMAL: Successful implementation of any weight loss program for dogs requires active, on-going communication between the dog owner/caretaker and the veterinary professional treating the pet. It is important that the prescribing veterinarian maintains an active veterinarian-client-patient relationship with the dog and the dog owner/caretaker during all phases of therapy and proactively communicates about their role in making the program successful in the short as well as the long-term. When drug therapy such as SLENTROL is included in the program, this discussion may include, but may not be limited to:
- SLENTROL is not a cure for obesity. The decreased appetite experienced when dogs are treated with dirlotapide is only temporary and lasts no longer than 1-2 days beyond the cessation of therapy. Weight gain will occur if the amount of food offered is not limited at the time SLENTROL is discontinued.
- Successful, long-term weight management requires changes that extend beyond the period of drug therapy. To maintain the weight lost when treated with SLENTROL, the adjustments in dietary management as well as physical activity that were begun as part of the overall weight loss program must be continued by the owner after drug therapy is discontinued.
- SLENTROL decreases the food intake of the dog. A decrease in appetite and associated begging behavior can be expected with SLENTROL treatment. However, if total inappetence or anorexia is observed for more than one day, these signs should be reported to the prescribing veterinarian immediately.
- Almost 1 in 4 of dogs placed on SLENTROL therapy experienced occasional episodes of vomiting and diarrhea. In most cases these episodes lasted for one or two days. The vomiting occurred most often during the first month of treatment or within a week of a dose increase. If vomiting does occur it is recommended to continue dosing at the same dose volume, however, the time of day or method of administration (with or without food) may be changed. If vomiting is severe or lasts longer than 2 days, consult your veterinarian immediately and have your dog evaluated.
- If you notice vision impairment, SLENTROL treatment should be discontinued until a veterinarian is consulted.
CONTRAINDICATIONS: SLENTROL should not be used in cats. SLENTROL increases the risk of producing hepatic lipidosis during weight loss in obese cats. SLENTROL is not recommended for use in dogs currently receiving long-term corticosteroid therapy. Do not use in dogs with liver disease [see PRECAUTIONS].
WARNINGS: Not for use in humans. Keep this and all drugs out of reach of children.
Adverse reactions associated with humans ingesting dirlotapide include: abdominal distention, abdominal pain, diarrhea, flatulence, headache, increased serum transaminases, nausea, and vomiting.
SLENTROL may cause eye-irritation. If accidental eye exposure occurs, flush the eyes immediately with clean water.
In some cases aggression has been reported in dogs being treated with SLENTROL. In case of aggression SLENTROL treatment should be discontinued until a veterinarian is consulted.
Precautions
Safety in breeding, pregnant, or lactating dogs has not been established. Caution should be taken when considering any weight loss program in growing dogs, including treatment with SLENTROL. SLENTROL has not been evaluated in dogs less than 1 year of age.All dogs should undergo a thorough history and physical examination that includes laboratory tests to screen for underlying conditions. Pre-existing ophthalmic or endocrine diseases (i.e. retinopathies, hyperadrenocorticism), should be managed prior to use of SLENTROL. Safe use in dogs with endocrine or underlying disease has not been established. Dogs experiencing unexpected rapid weight loss should be evaluated for potential underlying medical causes.
SLENTROL may produce a mild to moderate elevation in serum hepatic transaminase activity. If the elevation in alanine aminotransferase (ALT) activity is mild, continue SLENTROL and monitor as needed. If there is a marked elevation in ALT activity above the normal reference range or there is a simultaneous increase in aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), or total bilirubin, discontinue treatment with SLENTROL. Elevations in hepatic transaminase activity usually decrease when SLENTROL is discontinued.
The safety of SLENTROL use in dogs has not been evaluated beyond 1 year.
Adverse Reactions
The adverse reactions associated with treatment with SLENTROL include vomiting, loose stools/diarrhea, lethargy, and anorexia. These adverse reactions were mainly observed during the first month of treatment or during the week after a dose increase. Vomiting was usually mild in severity, of short duration, and resolved with continued SLENTROL treatment. The SLENTROL-treated dogs generally had an increased frequency and duration of vomiting and diarrhea compared to the control dogs. The control dogs received corn oil.
Adverse Reactions During Weight Loss:
|
Treatment |
Percentage of Patients with Reported Signs |
|
|
Control n = 88 |
SLENTROL n = 170 |
|
|
Vomiting |
21.6% |
24.7% |
|
Diarrhea |
6.8% |
12.4% |
|
Lethargy |
3.4% |
9.4% |
|
Anorexia |
2.3% |
7.6% |
|
Constipation |
1.1% |
2.4% |
|
Dehydration |
0% |
1.2% |
In addition to the adverse reactions listed above, there were other abnormal findings. Many control and SLENTROL-treated dogs had dental disease, abnormal skin and ear findings, and lameness/arthritis. The incidence of these findings were similar in both control and SLENTROL treated groups and most dogs had similar lesions noted pre-treatment. Two dogs in the SLENTROL treatment group developed corneal ulcers. One SLENTROL-treated and one control dog developed signs consistent with pancreatitis. One treated dog developed inappropriate urination and defecation and another treated dog developed polyuria and polydipsia.
A 5 year old Beagle with no medical history of seizures in the SLENTROL treatment group had a seizure on Day 52 of the study. The dog continued to receive SLENTROL until additional seizures occurred 11 and 12 days later. The investigator referred the case to a neurologist and the seizures continued approximately twice weekly. The neurologist found no lesions that support the causality of the seizures.
A 5 year old Dachshund developed a hepatopathy after 82 days of treatment and was withdrawn from the study for vomiting, increased hepatic enzymes, and anorexia. Vomiting continued for a few days after stopping treatment and the dog was hospitalized due to the anorexia. ALT activity levels continued to rise after all clinical observations resolved.
During weight stabilization, vomiting (16.1%) and lethargy (4.8%) were the most frequent adverse reactions associated with treatment with SLENTROL. Other adverse reactions included diarrhea (1.6%), anorexia (1.6%), and ataxia (1.6%).
In the post-treatment period, a 6 year old spayed female Chihuahua, was found dead by the owner 7 days after stopping dirlotapide therapy. The cause of death was not conclusive but did not appear to be related to the dirlotapide therapy.
Some dogs treated with SLENTROL displayed a mild to moderate elevation in serum hepatic transaminase activity early in treatment that decreased over time while treatment continued. Hepatic transaminases generally returned to normal when treatment was discontinued (See Precautions for further information).
Serum Chemistry Results:
|
Serum Analyte |
Percentage of Dogs |
|||
|
Control n = 88 |
SLENTROL n = 170 |
|||
|
Pred |
Poste |
Pred |
Poste |
|
|
ALTa > |
3.4% |
6.0% |
4.7% |
9.9% |
|
ASTb > |
0% |
4.8% |
3.5% |
9.2% |
|
ALPc > |
11.4% |
16.9% |
17.6% |
9.9% |
|
Cholesterol > |
14.8% |
9.6% |
14.7% |
4.6% |
a ALT = serum alanine aminotransferase activity,
b AST = serum aspartate aminotransferase activity,
c ALP = serum alkaline phosphatase activity. Dogs with ALP activity > 325 IU/L were excluded from the study.
d Pre = % of dogs with values above the laboratory reference range at pre-treatment.
e Post = % of dogs with values above the laboratory reference range after 4 months of treatment.
POST APPROVAL EXPERIENCE (March 2013):
The following adverse reactions are based on voluntary, post approval reporting. Not all adverse reactions are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data. The categories of adverse reactions are listed in decreasing order of frequency by body system.
Gastrointestinal: Vomiting, anorexia, diarrhea, polyphagia, bloody diarrhea, abdominal distension, lipase elevation, pancreatitis, hypersalivation, constipation, flatulence.
Behavioral: Lethargy, aggression, vocalization, nervousness, pica.
Hepatic: ALT elevation, ALP elevation, GGT elevation, low albumin, bilirubinemia.
Neurological: Convulsions.
Renal/Urinary: Polyuria, polydipsia, glucosuria.
Sensory: Ophthalmic disorders (including blindness).
Hematological: Leukophilia, neutrophilia, hyperglycemia.
Respiratory: Panting, dyspnea.
Dermatological: Alopecia, dermatitis.
General body system: Fever, death, dehydration.
To report a suspected adverse reaction call Zoetis Inc. at 1-888-963-8471.
For a copy of the Material Safety Data Sheet (MSDS) for SLENTROL oral solution call 1-888-963-8471.
Clinical Pharmacology
SLENTROL (dirlotapide) is a selective microsomal triglyceride transfer protein inhibitor that blocks the assembly and release of lipoproteins into the bloodstream. The mechanism of action for producing weight loss is not completely understood, but it seems to result from reduced fat absorption and a satiety signal from lipid-filled enterocytes.
SLENTROL mainly acts locally in the gut to reduce appetite, increase fecal fat and produce weight loss in the management of obesity in dogs. Dirlotapide is available systemically, but absorption in dogs is highly variable. Absorbed SLENTROL is metabolized in the liver. Dirlotapide and its metabolites are secreted in the bile and may undergo enterohepatic circulation. The fecal and biliary routes are the predominant routes of elimination. Dirlotapide in circulation is highly protein bound.
Although systemic blood levels do not directly correlate with effectiveness (effectiveness has been linked to drug concentrations in the gut), they seem to correlate with the systemic toxicity observed for this drug. Non-linear pharmacokinetics with less-than-proportional exposure, drug accumulation (at higher doses), and large inter-individual variability has been observed in multiple studies and at various dose levels. The mean elimination half-life ranged between 5 and 18 hours, and it seemed to increase with dose and with repeated dosing.
Effectiveness
The effectiveness of SLENTROL for the management of obesity was confirmed in two controlled, multi-site field studies using client-owned dogs. The control dogs received corn oil. More than 65 different pure breeds and mixed breed dogs were represented in the 276 dogs receiving SLENTROL during the clinical field studies. SLENTROL was evaluated in dogs receiving 135 other commonly used veterinary products such as vaccines, anthelmintics, antiparasitics, antimicrobials, collars, shampoos, dips, short-acting oral steroid preparations, and otic, ophthalmic, and topical steroid preparations. SLENTROL was not tested concomitantly with long-acting steroid products, anabolic steroids, or other products known to affect appetite.
In one field study evaluated for weight loss only, SLENTROL was effective in producing ≥0.7% weekly (≥0.1% daily) weight loss at an initial dosage 0.023 mg/lb (0.05 mg/kg), doubled at 14 days, and then adjusted monthly for 4 months. Two hundred and fifty eight (88 control and 170 SLENTROL) obese dogs, from 23 veterinary clinics, 21 in the US and 2 in Canada, with a body condition score (BCS) ≥8 on a 9-point scale2, participated in the study. SLENTROL-treated dogs lost a statistically significant (P≤ 0.0001) 11.8% body weight and 39% lost ≥ 13% body weight, an amount that has been shown to provide a health benefit in obese dogs3. At the end of treatment the final mean dosage was 0.12 mg/lb with a range of 0.05 mg/lb to 0.24 mg/lb (0.26 mg/kg, range 0.11 to 0.56 mg/kg) based on current body weight.
In a separate study, conducted at 14 different US veterinary clinics, 63 dogs that completed 4 months of SLENTROL treatment for weight loss were evaluated for weight management during a 3 month retraining phase. The weight management period was designed to educate the owner on the optimal amount of food necessary and exercise required to maintain the dog’s desired body weight. SLENTROL dosage was adjusted monthly (50% first adjustment and 25% subsequently) to maintain the desired body weight ± 5%.
Daily oral treatment with SLENTROL was safe and effectively stabilized body weight ± 5%, when adjusted monthly by 50% the first month and then by 25% monthly, as needed based on individual body weight changes. Vomiting and lethargy still occurred during the weight management phase.
At the completion of the weight management phase, SLENTROL was discontinued and body weight measured for an additional 2 months. Dogs regained approximately 3% of their body weight in 2 months, primarily during the first month after treatment was discontinued (n = 51).
Polyphagia was reported as an abnormal clinical finding in 8 of 106 dogs when SLENTROL was discontinued.
ANIMAL SAFETY:
Margin of Safety: In a controlled laboratory margin of safety study in neutered, obese Beagle dogs, SLENTROL (dirlotapide) was administered orally at 0, 0.5, 1.5 and 2.5 mg/kg once daily for 90 days. The control used was medium chain triglyceride oil.
Clinical Observations: Vomiting and loose stools were the most frequent clinical signs observed. Vomiting was dose-related and was observed in all treatment groups. Vomiting tended to occur within 3 hours of dosing and was more frequent during the first two to four weeks of treatment. Sporadic episodes of loose stools occurred throughout the 3-month dosing period in all dose groups. SLENTROL administration also resulted in a decrease in body weight, body condition score, and food intake in the treated dogs.
Clinical Chemistry: Dogs treated with SLENTROL revealed a dose-related decrease in serum cholesterol and high-density lipoprotein (HDL) concentration. Mean ALT activity and AST activity were increased at doses of 1.5 and 2.5 mg/kg/day. At 1 month, mean values of the mid and high dose-groups (versus control values) were ~2- to 4-fold and ~10- to 12-fold higher than controls for AST and ALT activity, respectively. The increases in ALT activity diminished during 3 months of continued treatment and were generally within normal limits for the 1.5 mg/kg/day group and ~5- to 6-fold higher than control values in the 2.5 mg/kg/day groups. The alkaline phosphatase levels mildly decreased in the treated dogs. Decreases in plasma concentrations of vitamins A and E were observed early in treatment for all SLENTROL-treated groups. Other effects included decreased blood urea nitrogen, total proteins, albumin, globulin, and calcium. All treatment-related clinical chemistry changes had reverted to normal at the end of the 1-month recovery phase.
Pathology: On gross necropsy, the mucosal surface of the small intestine appeared pale (presumably unabsorbed fat) primarily in the high dose groups. Enterocytes in the villus tips of the small intestine contained lipid vacuoles.
Individual Responses: In the high dose (2.5 mg/kg) group, two of six dogs (one male and one female) had AST elevations >100 U/L in combination with ALT elevations >500 U/L and a mild increase in bile acids. In the female, there were also mild increases in gamma-glutamyltransferase activity (GGT) and alkaline phosphatase (ALP) activity in the first month of treatment. These elevations decreased over time with continued treatment.
Acute Tolerance: In a separate 14-day acute tolerance study the safety of SLENTROL (dirlotapide) was evaluated following daily oral administration of 0, 2.5, 5.0 and 10 mg/kg body weight in normal weight Beagle dogs. The control used was medium chain triglyceride oil.
Clinical Observations: Vomiting was observed in all groups. Vomiting generally occurred within 4 hours of dosing and had the highest incidence during the first three days of treatment. The incidence of loose stools was lower than the incidence of vomiting, occurred intermittently throughout the study, and was also similar between the treated and control groups. SLENTROL administration produced reductions in food intake, organ weights, and body weight.
Clinical Chemistry & Histopathology: Changes in serum chemistry included a significant dose-related decrease in mean serum cholesterol and high-density lipoprotein, a non-dose-dependent mild to moderate elevation in serum hepatic transaminase enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, and mean decreases in serum total protein and albumin. Histopathology revealed accumulation of lipid vacuoles in the apical third of enterocytes in the small intestine in all treated dogs and minimal to mild periportal fatty change in the liver in five treated dogs.
Individual Responses: After 14 days of treatment, one high-dose female had an elevation in both ALT activity (257 U/L (~9-fold pre-treatment value) and AST activity (99 U/L (~4-fold pre-treatment value) and one mid-dose female had mild increases in ALT activity (102 U/L (~3-fold pre-treatment value), AST activity (71 U/L (~2-fold pre-treatment value) in combination with a moderate increase in bile acids (57.0 uM/L (~5-fold pre-treatment value). In the remaining dogs elevated hepatic transaminase enzymes were not accompanied by increases in other liver function indicators such as ALP, GGT, or total bilirubin.
1-Year use-dose study: In a separate long-term laboratory study, the efficacy and safety of SLENTROL (dirlotapide) was evaluated following daily oral administration of an initial dosage of 0.009 mL/lb medium chain triglyceride oil (control) or 0.1 mg/kg (dirlotapide) for 12 months in seventy-two obese Labrador Retrievers. The dosage was adjusted monthly to produce ≥ 1% weekly body weight loss for 6 months and then adjusted to maintain body weight constant (retraining or weight stabilization phase) for the next 6 months, up to a maximum dosage of 0.2 mL/kg (1 mg/kg) current body weight.
Clinical Observations: Diarrhea, vomiting, and excessive salivation were observed more frequently in SLENTROL-treated dogs than in control-treated dogs. Diarrhea was mild and observed intermittently throughout the study. Most episodes were of short duration and most dogs had only one or two episodes of diarrhea during the 12 months of treatment. Vomiting was also mild and the majority was observed during the first month of treatment. Excessive salivation was noted in approximately 4 of 48 SLENTROL-treated dogs, primarily during the first 28 days of treatment. Some treated dogs had ocular lesions at the end of the study including retinal degeneration and cataracts. Based on the available safety data, these lesions were considered incidental to the Labrador breed.
Clinical Chemistry: Serum cholesterol concentrations were significantly decreased during the first 6 months of treatment at the weight loss dosage and were at the low normal or below the laboratory reference range (135 to 270 mg/dL). Mean serum triglyceride concentration was not changed. A mild increase in mean ALT and AST activity was observed, which remained well within the published reference range for these analytes (ALT: 2 to 102 U/L, AST: 23 to 66 U/L). ALT activity tended to gradually increase as the SLENTROL dose increased during the first 6 months of weight loss and then decreased during the next 6 months despite continued SLENTROL treatment at the weight stabilization dosage. Other clinical pathology changes included low total proteins, low serum albumin and globulins, low blood urea nitrogen and low serum creatinine compared to control dogs. Mean and individual values were usually within the published normal range. Gamma glutamyl transferase, and total bilirubin were not changed during 12 months of SLENTROL treatment. Other abnormal clinical pathology values included sporadic findings that were similar in the control dogs, were not progressive with continued treatment, and returned to normal concentrations during a one-month post treatment period.
Individual Responses: A total of 10 of 48 dogs had sporadic mild to moderate ALT measurements that exceeded the upper limit of the reference range at some point during 12 months (maximum of 366 U/L) of SLENTROL treatment. The ALT elevations were not sustained despite continued treatment during the 6 months of weight stabilization. AST activity was marginally elevated in 1 of 48 dogs (maximum 84 U/L in a SLENTROL-treated dog and 116 U/L in a control dog) during 12 months of treatment. One dog, excluded from the totals, had a consistently elevated ALT and also had an elevated alkaline phosphatase prior to treatment. Except in this dog, no other changes in other liver function indicators accompanied these mild elevations in hepatic transaminases.
Fat Soluble Vitamins: During the first 6 months of treatment, plasma vitamin A and E concentrations of the SLENTROL-treated dogs were significantly below the vitamin A and E concentrations of the control dogs. Plasma vitamin A concentration was low after one month and the median values did not decline any further. Plasma vitamin E concentrations were lowest after 6 months of SLENTROL treatment but adipose tissue levels of vitamin E appeared to be increased compared to control dogs after 12 months of treatment. Plasma vitamin A and E concentrations appeared to increase during the weight stabilization phase (second 6 months of treatment) and returned to concentrations similar to the control dogs when SLENTROL treatment was discontinued. Prothrombin times were similar in the SLENTROL-treated and the control dogs and there were no clinical signs of abnormal hemostasis observed during the 12-month study.
STORAGE INFORMATION:
Store in original container at room temperature 15° to 30° C (59° to 86° F).
How Supplied
SLENTROL is available in 20, 50 and 150 mL bottles containing 5 mg/mL of dirlotapide in solution.
References
1 Wetterau, J.R. Microsomal triglyceride transfer protein: role in the assembly of intestinal lipoproteins. Intest. Lipid Metab. 2001; 171-184. Kluwer Academic/Plenum Publishers, New York, N.Y.
2 LaFlamme, D.P. Development and validation of a body condition scoring system for dogs. Canine Practice. 1997; 22:10-15.
3 Impellizeri, J.A., Tetrick, M.A., Muir, P. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis. J. Am. Vet. Med. Assoc. 2000, 216(7):1089-1091.
NADA #141-260, Approved by FDA
Distributed by: Zoetis Inc., Kalamazoo, MI 49007
Revised May 2013
PAA035167
CPN: 36902373
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
