Denamarin Chewable Tablets
This treatment applies to the following species:TWO ACTIVES - ONE PATENTED FORMULA
225 mg S-Adenosylmethionine* and 82 mg Silybin-phosphatidylcholine complex (SPC)
Available from your veterinarian
FOR DOGS OF ALL SIZES
Denamarin, a patented liver health supplement for veterinary use only, combines the pure and stabilized salt of S-Adenosylmethionine with silybin-phosphatidylcholine complex in a scored chewable tablet. Denamarin chewable tablets for Dogs of all sizes contain 225 mg S-Adenosylmethionine and 82 mg Silybin-phosphatidylcholine complex (SPC), providing 24 mg of Silybin A+B. While S-Adenosylmethionine is sensitive to moisture, the specific NMXSS75® S-Adenosylmethionine found in Denamarin chewable tablets has been manufactured to maintain stability.
Background
S-Adenosylmethionine is an endogenous molecule synthesized by cells throughout the body and is formed from the amino acid methionine and ATP. It is an essential part of three major biochemical pathways: transmethylation, transsulfuration and aminopropylation. As part of these pathways, S-Adenosylmethionine is essential to all cells and is particularly important in hepatocytes because of their central role in metabolism. A deficiency of S-Adenosylmethionine, therefore, may initiate or contribute to abnormalities of cellular structure and function in the liver as well as many other body tissues, including the brain.1-4 Conversely, exogenous administration of S-Adenosylmethionine has been shown to result in improvements in hepatocellular function in both in vivo and in vitro studies, without cytotoxicity or significant side effects.1-3,5-8 Precursors of S-Adenosylmethionine do not have similar effects. Administration of methionine to animals with decreased liver function may not increase hepatic S-Adenosylmethionine levels and may be toxic.2 The best way to increase S-Adenosylmethionine levels in the body is by direct supplementation with S-Adenosylmethionine.
Silybin is a biologically active component of an extract from milk thistle known as silymarin,9 and its absorption is enhanced by phosphatidylcholine.10-13 Silybin/silymarin has been shown to have beneficial effects on liver function.14-16
Purpose
The combination of S-Adenosylmethionine and silybin in Denamarin provides a multi-faceted approach to liver support.
S-Adenosylmethionine has been shown to increase hepatic glutathione levels in cats and dogs.1,3 Glutathione is a potent antioxidant that protects hepatic cells from toxins and death. A study found that low liver glutathione concentrations are common in dogs and cats with decreased hepatobiliary function.17 Denamarin is recommended to improve hepatic glutathione levels in patients to help maintain and protect liver function. Denamarin may also be used in other areas of tissue oxidant injury and RBC fragility caused by certain toxins or drugs which are related to reduced glutathione concentrations.2 Denamarin’s mechanism of action, however, goes beyond increasing glutathione levels, in that S-Adenosylmethionine has also been shown to protect liver cells from cell death5 and may be useful in cell regeneration.2 A study has also shown that S-Adenosylmethionine may improve bile flow in cats.8
In addition to supporting liver health, Denamarin may also support brain health as S-Adenosylmethionine has been shown to function as a neuroprotective agent.4,18,19
Silybin/silymarin has many different mechanisms of action. Studies have shown that it protects against oxidative stress in multiple cell types and species.20-23 A study in cats showed that it increased intracellular glutathione levels in neutrophils and increased phagocytic function.21 Other research has shown that it promotes hepatocyte protein synthesis,24 a mechanism for liver cell regeneration; inhibits IL-1β-induced production of several mediators (PGE2, IL-8, and MCP-1) and translocation of NF-KB (nuclear factor-kappa B), a key signaling agent;25 inhibits leukotriene production,26,27 which can be beneficial as production of leukotrienes is a component of the inflammatory response; stimulates biliary flow and production of hepatoprotective bile salts (e.g., beta-muricholate and ursodeoxycholate);28 and increases levels of glutathione.29
In a study, silybin was shown to be protective in acute Amanita phalloides mushroom poisoning in dogs, where one-third of the untreated dogs died, while all dogs in the silybin-group lived. Silybin-group dogs also had lower bilirubin, AST, ALT, and ALP levels and improved prothrombin times compared to control dogs.15 In another report, the liver enzymes improved in five out of six dogs with 30 days of silymarin administration.16
Results from a clinical trial indicate that Denamarin acts as a hepatoprotectant in dogs undergoing chemotherapy with lomustine (CCNU).30
Pharmacokinetics
In a pharmacokinetic study with fasted dogs given a S-Adenosylmethionine chewable tablet versus an enteric coated S-Adenosylmethionine tablet, bioavailability was found to be comparable between the two formulations. The time course of uptake, however, was significantly more rapid and consistent with the chewable tablet compared to the enteric coated S-Adenosylmethionine tablet.31
Silybin has low bioavailability.10 Denamarin, therefore, has been specially formulated to address this issue. It contains silybin in a complex with soybean phosphatidylcholine, resulting in superior absorption and bioavailability compared to silymarin or silybin administration alone.10-13 A study in dogs showed plasma silybin levels more than four times higher with administration of a silybin-phosphatidylcholine complex (SPC) than the levels obtained with administration of silymarin alone (see figure 1 below).13 Studies in rats showed that administration of the silybin-phosphatidylcholine complex was capable of reaching effective intracellular levels in liver microsomes not achieved with silybin administration alone.20 The SPC has also been shown to be bioavailable in cats.32
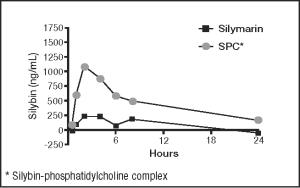
FIGURE 1
Plasma levels in dogs after oral administration of silymarin versus SPC (silybin-phosphatidylcholine complex). Each was given at the same amount based on silybin content (178 mg).13
Safety
The ingredients in Denamarin® possess exceptionally wide margins of safety. Oral acute toxicity studies in rats indicated an LD50 greater than 4,650 mg/kg for S-Adenosylmethionine.2 Clinically healthy dogs administered 20 mg/kg/day of S-Adenosylmethionine for 6 weeks and clinically healthy cats administered S-Adenosylmethionine at 2 times the recommended daily amount for 113 days remained healthy with no adverse effects from the administration.1,3
There are no known drug interactions or contraindications to the use of silybin/silymarin in animals.33,34 While mild side effects, such as gastrointestinal upset, itching and headache, have been rarely reported in primates,33,35,36 no side effects have been noted in dogs or cats.13,16,33,34,37,38 The specific silybin-phosphatidylcholine complex in Denamarin has been evaluated in both acute and chronic use safety studies: an acute toxicity study in dogs using levels greater than 80x the amount in Denamarin revealed no adverse physiologic effects,38 and a chronic toxicity study in monkeys who received greater than 80x the amount in Denamarin for 26 weeks showed no compound-related adverse effects.35
Administration As A Liver Health Supplement
Please follow the administration chart below as a guide.
For optimal absorption, the tablets should be given on an empty stomach, at least one hour before feeding, as the presence of food decreases the absorption of S-Adenosylmethionine. Denamarin can be used in conjunction with Marin® Plus for Dogs to provide additional silybin levels and the benefits of vitamin E, zinc, curcumin, and MCT oil supplementation. If Marin Plus is used in conjunction with Denamarin the two products should be administered 12 hours apart for best response.
Recommended Daily Administration
Denamarin Chewable Tablets
Contains 225 mg S-Adenosylmethionine* and 82 mg Silybin-phosphatidylcholine complex (SPC)
|
Weight (lbs.) |
# of tablets daily |
|
Up to 6 |
1/4 |
|
7 to 15 |
1/2 |
|
16 to 30 |
1 |
|
31 to 45 |
1 1/2 |
|
46 to 60 |
2 |
|
61 to 75 |
2 1/2 |
|
76 to 90 |
3 |
|
91 to 105 |
3 1/2 |
|
Over 105 |
4 |
*Denamarin Chewable Tablets contain NMXSS75® S-Adenosylmethionine, proprietary veterinary researched specifications.
Providing 24 mg of Silybin A+B.
Storage
Store in a cool dry place. Tablets are sensitive to moisture and extreme heat.
Keep lid tightly secured to ensure freshness.
Keep bottle out of reach of children.
This bottle may contain a non-toxic desiccant.
For additional information visit Denamarin.com.
Ask your veterinarian about Denamarin® for Cats.
REFERENCES
1. Center SA, Warner KL, McCabe J, et al. Am J Vet Res 2005;66:330-341.
2. Center SA. In Proceedings. 18th Annual ACVIM Forum 2000;550-552.
3. Center SA, Randolph JF, Warner K, et al. J Vet Intern Med 2005;19:303-314.
4. Tchantchou F, Graves M, Ortiz D, et al. J Nutr Health Aging 2006;10(6):541-544.
5. Webster CRL, Boria P, Usechak P, et al. Vet Therapeutics 2002;3:474-484.
6. Wallace KP, Center SA, Hickford FH, et al. J Am Anim Hosp Assoc 2002;38:246-254.
7. Webb CB, Twedt DC, Fettman MJ, et al. J Fel Med & Surg 2003;5:69-75.
8. Center SA, Warner KL. In Proceedings. 22nd Annual ACVIM Forum 2004;867-868.
9. Kvasnicka F, Biba B, Sevcik R, et al. J Chromatogr A 2003;990:239-245.
10. Morazzoni P, Magistretti MJ, Giachetti C, et al. Eur J Drug Metab Pharmacokinet 1992;17:39-44.
11. Schandalik R, Gatti G, Perucca E. Arzneimittelforschung 1992;42:964-968.
12. Barzaghi N, Crema F, Gatti G, et al. Eur J Drug Metab Pharmacokinet 1990;15:333-338.
13. Filburn CR, Kettenacker R, Griffin DW. J Vet Pharmacol Therap 2007;30:132-138.
14. Center SA. Vet Clin North Am Small Anim Pract 2004;34:67-172.
15. Vogel G, Tuchweber B, Trost W, et al. Toxicol Appl Pharmacol 1984;73:355-362.
16. Bontempo V, Bellucci D, Tonini B, et al. Obiettivi & Documenti Veterinari 2003;9:31-37.
17. Center SA, Warner KL, Erb HN. Am J Vet Res 2002;63:1187-1197.
18. Tchantchou F, Graves M, Falcone D, Shea TB. J Alzheimers Dis 2008;14:323-328.
19. Shea TB, Chan A. J Alzheimers Dis 2008;13:67-70.
20. Comoglio A, Leonarduzzi G, Carini R, et al. Free Radic Res Commun 1990;11:109-115.
21. Webb CB, McCord KW, Twedt DC. Am J Vet Res 2009;70(1):57-62.
22. Maran BA, Snow JE, Robinson JM, et al. J Vet Intern Med 2009;23(3):709-710 (abstract #72).
23. Dycus DL, Au AY, Grzanna MW, Frondoza CG. J Vet Intern Med 2008;22:813 (abstract #365).
24. Sonnenbichler J, Zetl I. Hoppe Seylers Z Physiol Chem 1984;365:555-566.
25. Au AY, Hasenwinkel JM, Frondoza CG. J Vet Pharmacol Ther 2011;34(2):120-129.
26. Dehmlow C, Erhard J, de Groot H. Hepatology 1996;23:749-754.
27. Dehmlow C, Murawski N, de Groot H. Life Sci 1996;58:1591-1600.
28. Crocenzi FA, Pellegrino JM, Sanchez Pozzi EJ, et al. Biochem Pharmacol 2000; 59:1015-1022.
29. Valenzuela A, Aspillaga M, Vial S, et al. Planta Med 1989;55:420-422.
30. Skorupski KA, Hammond GM, Irish AM, et al. J Vet Intern Med 2011;25:838-845.
31. Griffin DW, Whalen MO, Filburn CR. J Vet Intern Med 2009;22(3):767 (abstract #284).
32. Webb CB, Gustafson D, Twedt DC. Intern J Appl Res Vet Med 2012;10(2):107-112.
33. Sartor LL, Trepanier LA. Compend Contin Educ Pract Vet 2003;25:432-447.
34. Minton J. Compend Contin Educ Pract Vet 2004;26:631-632.
35. Data on file. Nutramax Laboratories, Inc., Edgewood, MD 21040, USA 1991.
36. Flatland B. Compend Contin Educ Pract Vet 2003;25:514-524.
37. Filburn CR, Kettenacker R, Griffin DW. Intern J Appl Res Vet Med 2006;4(4):326-334.
38. Data on file. Nutramax Laboratories, Inc., Edgewood, MD 21040, USA 1989.
Visit NutramaxLabs.com for current specials and promotions.
U.S. Patent Nos. 6,555,141; 6,863,906 & 7,563,779
Proverbs 12:10
love YOUR PET
trust YOUR VET®
LYPTYV.com
MADE IN USA FROM GLOBALLY SOURCED INGREDIENTS
nutramax LABORATORIES, VETERINARY SCIENCES, INC., 946 Quality Drive, Lancaster, SC 29720
nutramaxlabs.com
1-888-886-6442
02.1029.12
IN-00039
|
30 CHEWABLE TABLETS |
01.1076.10 / CT-00089 00.1100.09 |
|
75 CHEWABLE TABLETS |
01.1077.10 / CT-00090 00.1101.09 |
CPN: 1291034.7
946 QUALITY DRIVE, LANCASTER, SC, 29720
| Telephone: | 803-289-6000 | |
| Toll-Free: | 800-925-5187 | |
| Fax: | 803-283-3073 | |
| Website: | www.nutramaxlabs.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
