Clindamycin Palmitate Hydrochloride Granules Prescribing Information
Package insert / product label
Dosage form: granules for oral solution
Drug class: Lincomycin derivatives
Medically reviewed by Drugs.com. Last updated on May 6, 2024.
On This Page
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin palmitate hydrochloride for oral solution and other antibacterial drugs, clindamycin palmitate hydrochloride for oral solution should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Not for Injection
WARNING
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
Because clindamycin therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate, as described in the INDICATIONS AND USAGE section. It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Related/similar drugs
prednisone, amoxicillin, doxycycline, ciprofloxacin, cephalexin, azithromycin, metronidazole
Clindamycin Palmitate Hydrochloride Granules Description
Clindamycin palmitate hydrochloride, USP is a water soluble hydrochloride salt of the ester of clindamycin and palmitic acid. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compound lincomycin. The structural formula is represented below:
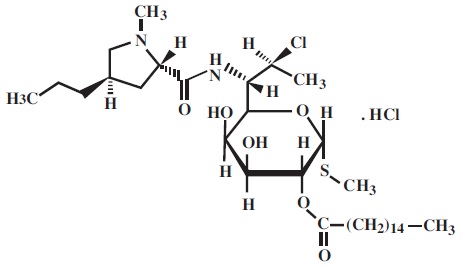
The chemical name for clindamycin palmitate hydrochloride is Methyl 7-chloro-6, 7, 8- trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D- galacto-octopyranoside 2-palmitate monohydrochloride.
Clindamycin palmitate hydrochloride for oral solution, USP (Pediatric) is flavored granules containing clindamycin palmitate hydrochloride, USP for reconstitution. Each 5 mL contains the equivalent of 75 mg clindamycin. Inactive ingredients: artificial cherry flavor, dextrin, ethylparaben, poloxamer 188, sucrose.
Clindamycin Palmitate Hydrochloride Granules - Clinical Pharmacology
Human Pharmacology
Absorption
Pharmacokinetic studies comparing clindamycin palmitate HCl with clindamycin hydrochloride show that both drugs reach their peak active serum concentrations at the same time, indicating a rapid hydrolysis of the palmitate to the clindamycin.
Pharmacokinetic studies with clindamycin palmitate HCl in normal pediatric patients weighing 50 lbs to 100 lbs given 2 mg/kg, 3 mg/kg or 4 mg/kg every 6 hours (8, 12 or 16 mg/kg/day) demonstrated mean peak clindamycin serum concentrations of 1.24 mcg/mL, 2.25 mcg/mL and 2.44 mcg/mL respectively, one hour after the first dose. By the fifth dose, the 6-hour serum concentration had reached equilibrium. Peak serum concentrations after this time would be about 2.46 mcg/mL, 2.98 mcg/mL and 3.79 mcg/mL with doses of 8, 12 and 16 mg/kg/day, respectively. Serum concentrations have been uniform and predictable from person to person and dose to dose.
Distribution
Multiple-dose studies in neonates and infants up to 6 months of age show that the drug does not accumulate in the serum and is excreted rapidly. Serum concentrations exceed the MICs for most indicated organisms for at least six hours following administration of the usually recommended doses of clindamycin palmitate hydrochloride for oral solution in adults and pediatric patients. Clindamycin is widely distributed in body fluids and tissues (including bones).
No significant concentrations of clindamycin are attained in the cerebrospinal fluid, even in the presence of inflamed meninges.
Metabolism
In vitro studies in human liver and intestinal microsomes indicated that clindamycin is predominantly metabolized by Cytochrome P450 3A4 (CYP3A4), with minor contribution from CYP3A5, to form clindamycin sulfoxide and a minor metabolite, N-desmethylclindamycin.
Excretion
Approximately 10% of the bioactivity is excreted in the urine and 3.6% in the feces; the remainder is excreted as bioinactive metabolites.
The average serum half-life after doses of clindamycin palmitate hydrochloride for oral solution is approximately two hours in pediatric patients.
Specific Populations
Patients with Renal/Hepatic Impairment
The elimination half-life of clindamycin is increased slightly in patients with markedly reduced renal or hepatic function. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum. Dosage schedules do not need to be modified in patients with renal or hepatic disease.
Elderly Patients
Pharmacokinetic studies in elderly volunteers (61 to 79 years) and younger adults (18 to 39 years) indicate that age alone does not alter clindamycin pharmacokinetics (clearance, elimination half-life, volume of distribution, and area under the serum concentration-time curve) after intravenous administration of clindamycin phosphate. After oral administration of clindamycin hydrochloride, the average elimination half-life is increased to approximately 4.0 hours (range 3.4 to 5.1 h) in the elderly compared to 3.2 hours (range 2.1 to 4.2 h) in younger adults; administration of clindamycin palmitate HCl resulted in a similar elimination half-life value of about 4.5 hours in elderly subjects. However, the extent of absorption is not different between age groups and no dosage alteration is necessary for the elderly with normal hepatic function and normal (age-adjusted) renal function1.
Obese Pediatric Patients Aged 2 to Less than 18 Years and Obese Adults Aged 18 to 20 Years
An analysis of pharmacokinetic data in obese pediatric patients aged 2 to less than 18 years and obese adults aged 18 to 20 years demonstrated that clindamycin clearance and volume of distribution, normalized by total body weight, are comparable regardless of obesity.
Microbiology
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is bacteriostatic.
Resistance
Resistance to clindamycin is most often caused by modification of specific bases of the 23S ribosomal RNA. Cross-resistance between clindamycin and lincomycin is complete. Because the binding sites for these antibacterial drugs overlap, cross-resistance is sometimes observed among lincosamides, macrolides and streptogramin B. Macrolide-inducible resistance to clindamycin occurs in some isolates of macrolide-resistant bacteria. Macrolide-resistant isolates of staphylococci and beta-hemolytic streptococci should be screened for induction of clindamycin resistance using the D-zone test.
Antimicrobial Activity
Clindamycin has been shown to be active against most of the isolates of the following microorganisms, both in vitro and in clinical infections (see INDICATIONS AND USAGE).
Gram-positive bacteria
Staphylococcus aureus (methicillin-susceptible strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyogenes
Anaerobic bacteria
Clostridium perfringens
Fusobacterium necrophorum
Fusobacterium nucleatum
Peptostreptococcus anaerobius
Prevotella melaninogenica
The following in vitro data are available, but their clinical significance is unknown. At least 90% of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for clindamycin against isolates of a similar genus or organism group. However, the efficacy of clindamycin in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria
Staphylococcus epidermidis (methicillin-susceptible strains)
Streptococcus agalactiae
Streptococcus anginosus
Streptococcus mitis
Streptococcus oralis
Anaerobic bacteria
Actinomyces israelii
Clostridium clostridioforme
Eggerthella lenta
Finegoldia (Peptostreptococcus) magna
Micromonas (Peptostreptococcus) micros
Prevotella bivia
Prevotella intermedia
Cutibacterium acnes
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Indications and Usage for Clindamycin Palmitate Hydrochloride Granules
Clindamycin palmitate hydrochloride for oral solution is indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. Clindamycin is also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, as described in the BOXED WARNING, before selecting clindamycin the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin).
Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, nongonococcal tubo-ovarian abscess, pelvic cellulitis and postsurgical vaginal cuff infection.
Streptococci: Serious respiratory tract infections; serious skin and soft tissue infections.
Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections.
Pneumococci: Serious respiratory tract infections. Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin palmitate hydrochloride for oral solution and other antibacterial drugs, clindamycin palmitate hydrochloride for oral solution should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
This drug is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin.
Warnings
See BOXED WARNING.
Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin palmitate hydrochloride, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Anaphylactic and Severe Hypersensitivity Reactions
Anaphylactic shock and anaphylactic reactions have been reported (see ADVERSE REACTIONS).
Severe hypersensitivity reactions, including severe skin reactions such as toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson syndrome (SJS), some with fatal outcome, have been reported (see ADVERSE REACTIONS).
In case of such an anaphylactic or severe hypersensitivity reaction, discontinue treatment permanently and institute appropriate therapy.
A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
Nephrotoxicity
Clindamycin is potentially nephrotoxic and cases with acute kidney injury have been reported. Consider monitoring of renal function particularly in patients with pre-existing renal dysfunction or those taking concomitant nephrotoxic drugs. In case of acute kidney injury, discontinue clindamycin palmitate hydrochloride for oral solution when no other etiology is identified.
Usage in Meningitis: Since clindamycin does not diffuse adequately into the cerebrospinal fluid, the drug should not be used in the treatment of meningitis.
Precautions
General
Review of experience to date suggests that a subgroup of older patients with associated severe illness may tolerate diarrhea less well. When clindamycin is indicated in these patients, they should be carefully monitored for change in bowel frequency.
Clindamycin palmitate hydrochloride for oral solution should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis. Clindamycin palmitate hydrochloride for oral solution should be prescribed with caution in atopic individuals.
Indicated surgical procedures should be performed in conjunction with antibiotic therapy.
The use of clindamycin palmitate hydrochloride for oral solution occasionally results in overgrowth of nonsusceptible organisms-particularly yeasts. Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation.
Clindamycin dosage modification is not necessary in patients with renal disease. In patients with moderate to severe liver disease, prolongation of clindamycin half-life has been found. However, it was postulated from studies that when given every eight hours, accumulation should rarely occur. Therefore, dosage modification in patients with liver disease may not be necessary. However, periodic liver enzyme determinations should be made when treating patients with severe liver disease.
Prescribing clindamycin palmitate hydrochloride for oral solution in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs including clindamycin palmitate hydrochloride for oral solution should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clindamycin palmitate hydrochloride for oral solution is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clindamycin palmitate hydrochloride for oral solution or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
During prolonged therapy, periodic liver and kidney function tests and blood counts should be performed.
Drug Interactions
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Clindamycin is metabolized predominantly by CYP3A4, and to a lesser extent by CYP3A5, to the major metabolite clindamycin sulfoxide and minor metabolite N-desmethylclindamycin. Therefore inhibitors of CYP3A4 and CYP3A5 may increase plasma concentrations of clindamycin and inducers of these isoenzymes may reduce plasma concentrations of clindamycin. In the presence of strong CYP3A4 inhibitors, monitor for adverse reactions. In the presence of strong CYP3A4 inducers such as rifampicin, monitor for loss of effectiveness.
In vitro studies indicate that clindamycin does not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2E1 or CYP2D6 and only moderately inhibits CYP3A4.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Fertility studies in rats treated orally with up to 300 mg/kg/day (approximately 1.6 times the highest recommended adult human oral dose based on mg/m2) revealed no effects on fertility or mating ability.
Pregnancy: Teratogenic Effects
In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of congenital abnormalities.
Clindamycin should be used during the first trimester of pregnancy only if clearly needed. There are no adequate and well-controlled studies in pregnant women during the first trimester of pregnancy. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
Reproduction studies performed in rats and mice using oral doses of clindamycin up to 600 mg/kg/day (3.2 and 1.6 times the highest recommended adult human dose based on mg/m2, respectively) or subcutaneous doses of clindamycin up to 250 mg/kg/day (1.3 and 0.7 times the highest recommended adult human dose based on mg/m2, respectively) revealed no evidence of teratogenicity.
Nursing Mothers
Limited published data based on breast milk sampling reports that clindamycin appears in human breast milk in the range of less than 0.5 to 3.8 mcg/mL. Clindamycin has the potential to cause adverse effects on the breast-fed infant's gastrointestinal flora. If oral or intravenous clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the breast-fed infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breast-fed child from clindamycin or from the underlying maternal condition.
Pediatric Use
When clindamycin palmitate hydrochloride for oral solution is administered to the pediatric population (birth to 16 years), appropriate monitoring of organ system functions is desirable.
Geriatric Use
Clinical studies of clindamycin did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients. However, other reported clinical experience indicates that antibiotic-associated colitis and diarrhea (due to Clostridioides difficile) seen in association with most antibiotics occur more frequently in the elderly (> 60 years) and may be more severe. These patients should be carefully monitored for the development of diarrhea.
Pharmacokinetic studies with clindamycin have shown no clinically important differences between young subjects (18 to 39 years) and elderly subjects (61 to 79 years) with normal hepatic function and normal (age-adjusted) renal function after oral or intravenous administration.
Adverse Reactions/Side Effects
The following reactions have been reported with the use of clindamycin.
Infections and infestations: Clostridioides difficile colitis
Gastrointestinal: Abdominal pain, pseudomembranous colitis, esophagitis, nausea, vomiting and diarrhea (see BOXED WARNING). The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see WARNINGS). An unpleasant or metallic taste has been reported after oral administration.
Hypersensitivity Reactions: Generalized mild to moderate morbilliform-like (maculopapular) skin rashes are the most frequently reported adverse reactions. Vesiculobullous rashes, as well as urticaria, have been observed during drug therapy. Severe skin reactions such as Toxic Epidermal Necrolysis, some with fatal outcome, have been reported (see WARNINGS). Cases of Acute Generalized Exanthematous Pustulosis (AGEP), erythema multiforme, some resembling Stevens-Johnson syndrome, anaphylactic shock, anaphylactic reaction and hypersensitivity have also been reported.
Skin and Mucous Membranes: Pruritus, vaginitis, angioedema, and rare instances of exfoliative dermatitis have been reported (see Hypersensitivity Reactions).
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Renal: Acute kidney injury (see WARNINGS).
Hematopoietic: Transient neutropenia (leukopenia) and eosinophilia have been reported. Reports of agranulocytosis and thrombocytopenia have been made. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of the foregoing.
Immune system: Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
Musculoskeletal: Cases of polyarthritis have been reported.
Overdosage
Significant mortality was observed in mice at an intravenous dose of 855 mg/kg and in rats at an oral or subcutaneous dose of approximately 2,618 mg/kg. In the mice, convulsions and depression were observed. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
Clindamycin Palmitate Hydrochloride Granules Dosage and Administration
If significant diarrhea occurs during therapy, this antibiotic should be discontinued (see BOXED WARNING).
Concomitant administration of food does not adversely affect the absorption of clindamycin palmitate HCl contained in clindamycin palmitate hydrochloride Flavored Granules.
Serious infections: 8 to 12 mg/kg/day (4 to 6 mg/lb/day) divided into 3 or 4 equal doses. Severe infections: 13 to 16 mg/kg/day (6.5 to 8 mg/lb/day) divided into 3 or 4 equal doses. More severe infections: 17 to 25 mg/kg/day (8.5 to 12.5 mg/lb/day) divided into 3 or 4 equal doses.
In pediatric patients weighing 10 kg or less, 1/2 teaspoon (37.5 mg) three times a day should be considered the minimum recommended dose.
Clindamycin should be dosed based on total body weight regardless of obesity.
Serious infections due to anaerobic bacteria are usually treated with clindamycin injection. However, in clinically appropriate circumstances, the physician may elect to initiate treatment or continue treatment with clindamycin palmitate hydrochloride for oral solution.
NOTE: In cases of β-hemolytic streptococcal infections, treatment should be continued for at least 10 days.
Reconstitution Instructions:
When reconstituted with water as follows, each 5 mL (teaspoon) of solution contains clindamycin palmitate HCl, USP equivalent to 75 mg clindamycin.
Reconstitute bottles of 100 mL with 75 mL of water. Add a large portion of the water and shake vigorously; add the remainder of the water and shake until the solution is uniform.
Storage Conditions:
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
How is Clindamycin Palmitate Hydrochloride Granules supplied
Clindamycin palmitate HCl for oral solution, USP (pediatric) is a white to off-white powder forming a clear colorless cherry flavored solution upon constitution with water.
When reconstituted as directed, each bottle yields 100 mL of solution containing 75 mg of clindamycin per 5 mL (NDC 53746-468-19).
Rx only
References
- Smith RB, Phillips JP: Evaluation of CLEOCIN HCl and CLEOCIN Phosphate in an Aged Population. Upjohn TR 8147-82-9122-021, December 1982.
Manufactured by:
Amneal Pharmaceuticals Pvt. Ltd.
Ahmedabad 382220, INDIA
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 12-2023-00
PRINCIPAL DISPLAY PANEL
NDC 53746-468-19
Clindamycin Palmitate Hydrochloride for Oral Solution, USP (Pediatric)
75 mg/5 mL
Bottle Label
Rx only
Amneal Pharmaceuticals LLC

NDC 53746-468-19
Clindamycin Palmitate Hydrochloride for Oral Solution, USP (Pediatric)
75 mg/5 mL
Carton
Rx only
Amneal Pharmaceuticals LLC

| CLINDAMYCIN PALMITATE HYDROCHLORIDE
(PEDIATRIC)
clindamycin palmitate hydrochloride powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amneal Pharmaceuticals of New York LLC (123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amneal Pharmaceuticals Private Limited | 915076126 | analysis(53746-468) , label(53746-468) , manufacture(53746-468) , pack(53746-468) | |
Frequently asked questions
- What are the best antibiotics for a tooth infection?
- What is the best antibiotic to treat strep throat?
- Is clindamycin a strong antibiotic?
- Can I take clindamycin if I am allergic to penicillin?
- Is clindamycin a penicillin?
- Why can't you lay down after taking clindamycin?
- Can clindamycin be used to treat chlamydia?
More about clindamycin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (804)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: lincomycin derivatives
- Breastfeeding
- En español
Patient resources
Professional resources
- Clindamycin monograph
- Clindamycin Capsules (FDA)
- Clindamycin Injection (FDA)
- Clindamycin Injection Concentrate (FDA)
- Clindamycin in Dextrose Injection (FDA)
Other brands
Cleocin, Cleocin Pediatric, Cleocin Phosphate

