Infergen: Package Insert / Prescribing Info
Package insert / product label
Generic name: interferon alfacon-1
Dosage form: injection
Drug class: Interferons
Medically reviewed by Drugs.com. Last updated on Mar 24, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
INFERGEN (interferon alfacon-1)
injection for subcutaneous use
Initial U.S. Approval: 1997
WARNING: FATAL OR LIFE-THREATENING DISORDERS AND RIBAVIRIN ASSOCIATED EFFECTS
See Full Prescribing Information for complete boxed warning
• May cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Monitor closely and withdraw therapy with persistently severe or worsening signs or symptoms of the above disorders. (5.2)
Use with Ribavirin
• Ribavirin may cause birth defects and fetal death; avoid pregnancy in female patients and female partners of male patients. (5.1)
• Ribavirin causes hemolytic anemia which may exacerbate cardiac disease (5.1)
Recent Major Changes
Boxed Warning 05/2013
Indications and Usage for Infergen
- •
- INFERGEN (interferon alfacon-1) is indicated for treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease. This indication is based on clinical trials conducted using INFERGEN alone at a time before combination treatment of chronic hepatitis C became the standard of care, and on a single trial evaluating INFERGEN in combination with ribavirin in patients who failed to respond to previous treatment with a pegylated interferon and ribavirin. Use of monotherapy with an interferon such as INFERGEN for the treatment of hepatitis C is not recommended unless a patient is unable to take ribavirin.
- •
- The safety and efficacy of the combination of INFERGEN/ribavirin in treatment-naïve patients or in patients co-infected with HBV or HIV-1 have not been evaluated.
- •
- Patients with the following characteristics are less likely to benefit from retreatment with INFERGEN/ribavirin combination therapy: response of <1 log10 drop HCV RNA on previous treatment, Genotype 1, high viral load (≥850,000 IU/mL), African American race, and/or presence of cirrhosis.
Infergen Dosage and Administration
- •
- INFERGEN is administered by subcutaneous injection.
- •
- Monotherapy: INFERGEN 9 mcg three times weekly for 24 weeks (as initial treatment) or 15 mcg three times weekly for up to 48 weeks (as retreatment). (2.1)
- •
- Combination treatment: INFERGEN 15 mcg daily with ribavirin 1,000 or 1,200 mg (for body weight < 75 kg and ≥ 75 kg) daily for up to 48 weeks (as retreatment). (2.2)
- •
- Dose reduction is recommended in patients experiencing serious adverse reactions. (2.3)
Dosage Forms and Strengths
Contraindications
- •
- hepatic decompensation (Child-Pugh score >6 [class B and C])
- •
- autoimmune hepatitis
- •
- known hypersensitivity reactions such as urticaria, angioedema, bronchoconstriction, anaphylaxis to interferon alphas or to any component of the product
Additional contraindications for combination therapy with ribavirin: (4)
- •
- women who are pregnant
- •
- men whose female partners are pregnant
- •
- patients with hemoglobinopathies (e.g., thalassemia major, sickle-cell anemia)
- •
- patients with creatinine clearance <50 mL/min
Warnings and Precautions
- •
- Birth defects and fetal death with ribavirin: Female patients must have a negative pregnancy test prior to therapy, use at least 2 forms of contraception, and undergo monthly pregnancy tests. (5.1)
Patients exhibiting any of the following conditions should be closely monitored and may require dose reduction or discontinuation of therapy:
- •
- Use with ribavirin (5.1)
- •
- Neuropsychiatric Disorders (5.2)
- •
- Cardiovascular Events (5.3)
- •
- Pulmonary Disorders (5.4)
- •
- Hepatic Failure(5.5)
- •
- Renal Insufficiency (5.6)
- •
- Cerebrovascular Disorders (5.7)
- •
- Bone Marrow Toxicity (5.8)
- •
- Colitis (5.9)
- •
- Pancreatitis (5.10)
- •
- Hypersensitivity (5.11)
- •
- Autoimmune Disorders (5.12)
- •
- Ophthalmologic Disorders (5.13)
- •
- Peripheral Neuropathy (5.14)
- •
- Endocrine Disorders (5.15)
- •
- Laboratory Tests (5.16)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence > 40%) are fatigue, fever, rigors, body pain, headache, abdominal pain, nausea, granulocytopenia, arthralgia, myalgia, back pain, neutropenia, and influenza-like illness. (6.1) (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or Kadmon Pharmaceuticals, LLC at 1-877-377-7862 or www.fda.gov/medwatch
Drug Interactions
Myelosuppressive drugs: Monitor closely for toxicity. (7.1)
Check for drug interactions known to occur with use of ribavirin.
Use In Specific Populations
- •
- Ribavirin Pregnancy Registry 1-800-593-2214 (8.1)
- •
- Nursing mothers (8.3)
- •
- Pediatrics: safety and efficacy have not been established (8.4)
- •
- Geriatrics: neuropsychiatric, cardiac, pulmonary, GI, and systemic (flu-like) adverse reactions may be more severe (8.5)
- •
- Hepatic Impairment: safety and efficacy have not been studied (8.6)
- •
- Renal Impairment: safety and efficacy have not been studied (8.7)
- •
- Organ transplant: safety and efficacy have not been studied (8.8)
- •
- HIV or HBV coinfection: safety and efficacy have not been studied (8.9)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2013
Full Prescribing Information
WARNING: FATAL OR LIFE-THREATENING DISORDERS
Alpha interferons, including INFERGEN, cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Patients with persistently severe or worsening symptoms of these conditions should be withdrawn from therapy. In many but not all cases, these disorders resolve after stopping interferon alfacon-1 therapy. [see WARNINGS AND PRECAUTIONS (5) and ADVERSE REACTIONS (6.1)].
Use with Ribavirin: Ribavirin may cause birth defects and/or death of the unborn child. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. Ribavirin causes hemolytic anemia. The anemia associated with ribavirin therapy may result in a worsening of cardiac disease. [see WARNINGS AND PRECAUTIONS (5); and Ribavirin Labeling].
1. Indications and Usage for Infergen
1.1 Chronic Hepatitis C
INFERGEN® (interferon alfacon-1) is indicated for treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease. This indication is based on clinical trials conducted using INFERGEN as monotherapy prior to the time that combination treatment was the standard of care and on a single trial evaluating INFERGEN in combination with ribavirin in patients who failed to respond to previous treatment with a pegylated interferon and ribavirin.
The following points should be considered when initiating treatment with INFERGEN:
- •
- Use of monotherapy with an interferon such as INFERGEN for the treatment of hepatitis C is not recommended unless a patient is unable to take ribavirin.
- •
- The safety and efficacy of the combination of INFERGEN/ribavirin in treatment-naïve patients or in patients co-infected with HBV or HIV-1 have not been evaluated.
- •
- Patients with the following characteristics are less likely to benefit from retreatment with combination therapy: response of <1 log10 drop HCV RNA on previous treatment, Genotype 1, high viral load (>850,000 IU/mL), African American race, and/or presence of cirrhosis.
- •
- No safety and efficacy data are available for treatment of longer than one year.
2. Infergen Dosage and Administration
2.1 INFERGEN Monotherapy Dosing
The recommended dose of INFERGEN monotherapy for the initial treatment of chronic HCV infection is 9 mcg administered three times a week as a single subcutaneous injection for 24 weeks [see Clinical Studies (14.1), Medication Guide for instructions].
The recommended dose of INFERGEN monotherapy for patients who tolerated previous interferon therapy and did not respond or relapsed following its discontinuation is 15 mcg administered three times a week as a single subcutaneous injection for up to 48 weeks [see Clinical Studies (14.2), Medication Guide for instructions]. Patients who do not tolerate initial standard interferon therapy should not be treated with INFERGEN therapy 15 mcg three times a week.
2.2 Combination Treatment with INFERGEN/Ribavirin Dosing
The recommended dose of INFERGEN is 15 mcg daily administered as a single subcutaneous injection in combination with weight-based ribavirin at 1,000 mg - 1,200 mg (< 75 kg and ≥75 kg) orally in two divided doses for up to 48 weeks. [see Clinical Studies (14.3), Medication Guide for instructions].
Ribavirin should be taken with food. INFERGEN/ribavirin should not be used in patients with creatinine clearance < 50 mL/min [see CONTRAINDICATIONS (4)].
2.3 Dose Modifications
If a serious adverse reaction develops during the course of treatment [see WARNINGS AND PRECAUTIONS (5)] discontinue or modify the dosage of INFERGEN and/or ribavirin until the adverse event abates or decreases in severity. If persistent or recurrent serious adverse events develop despite adequate dosage adjustment, discontinue treatment. Upon resolution or improvement of the adverse reaction, resuming INFERGEN and/or ribavirin may be considered.
INFERGEN Monotherapy Dose Modifications
Dose reduction to 7.5 mcg may be necessary following a serious adverse reaction. If serious adverse events continue to occur, dosing should be interrupted or discontinued as the efficacy of lower doses has not been established.
INFERGEN/Ribavirin Combination Therapy Dose Modifications
Stepwise dose reduction from 15 mcg to 9 mcg and from 9 mcg to 6 mcg may be necessary for serious adverse reactions.
Guidelines for INFERGEN/Ribavirin Dose Modifications
Tables 1, 2, and 3 provide guidelines for dose modifications and discontinuation of INFERGEN and/or ribavirin based on depression or laboratory parameters.
| * See DSM-IV for definitions. | |||||
|
Depression Severity* |
Initial Management (4–8 Weeks) |
Depression |
|||
|
|
Dose Modification |
Visit Schedule |
Remains Stable |
Improves |
Worsens |
|
Mild |
No change to INFERGEN dose or ribavirin dose. |
Evaluate once weekly by visit and/or phone. |
Continue weekly visit schedule. |
Resume normal visit schedule. |
(See moderate or severe depression) |
|
Moderate |
Decrease INFERGEN dose from 15 mcg to 9 mcg; or from 9 mcg to 6 mcg, no change to ribavirin dose. |
Evaluate once weekly (office visit at least every other week). |
Consider psychiatric consultation. Continue reduced dosing. |
If symptoms improve and are stable for 4 weeks, may resume normal visit schedule. Continue reduced INFERGEN dosing or return to normal INFERGEN dose. |
(See severe depression) |
|
Severe |
Discontinue INFERGEN and ribavirin permanently. |
Not applicable. |
Psychiatric therapy necessary. |
Not applicable. |
Not applicable. |
|
Laboratory Values |
Action |
|
ANC < 0.75 × 109/L |
Reduce INFERGEN dose from 15 mcg to 9 mcg, or from 9 mcg to 6 mcg; maintain ribavirin dose at 1200 mg or 1000 mg. |
|
ANC < 0.50 × 109/L |
INFERGEN and ribavirin treatment should be suspended until ANC values return to more than 1000/mm3. |
|
Platelet Count < 50 × 109/L |
Reduce INFERGEN dose from 15 mcg to 9 mcg or from 9 mcg to 6 mcg; maintain ribavirin dose at 1200 mg or 1000 mg. |
|
Platelet Count < 25 × 109/L |
INFERGEN and ribavirin treatment should be discontinued. |
| * For adult patients with a history of stable cardiac disease receiving INFERGEN in combination with ribavirin, the INFERGEN dose should be reduced from 15 mcg to 9 mcg or 9 mcg to 6 mcg and the ribavirin dose by 200 mg/day if a >2 g/dL decrease in hemoglobin is observed during any 4-week period. Both INFERGEN and ribavirin should be permanently discontinued if patients have hemoglobin levels <12 g/dL after this ribavirin dose reduction. ** 1st dose reduction of ribavirin is by 200 mg/day. 2nd dose reduction of ribavirin (if needed) is by an additional 200 mg/day. |
||||||||||
|
Condition |
INFERGEN |
Ribavirin |
||||||||
|
Hgb <10 g/dL |
History of Cardiac or Cerebrovascular Disease, reduce dose of INFERGEN |
Adjust dose** |
||||||||
|
Hgb <8.5 g/dL |
Permanently discontinue |
Permanently discontinue |
||||||||
Renal Function: INFERGEN/ribavirin should not be used in patients with creatinine clearance <50 mL/min. [See CONTRAINDICATIONS (4), WARNINGS AND PRECAUTIONS (5) and Ribavirin Labeling].
2.4 Discontinuation of Treatment
Patients who fail to achieve at least a 2 log10 drop at 12 weeks or undetectable HCV-RNA at week 24 are highly unlikely to achieve SVR and discontinuation of therapy should be considered [See Clinical Studies (14)].
Ribavirin should be discontinued in any patient who temporarily or permanently discontinues INFERGEN.
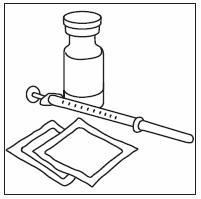
2.5 Preparation and Administration
Just prior to injection, INFERGEN may be allowed to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration; if particulates or discoloration are observed, the vial should not be used.
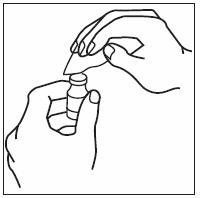
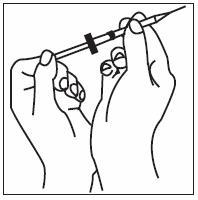
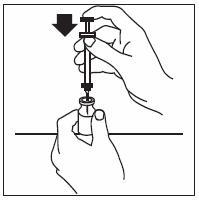
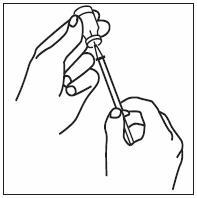
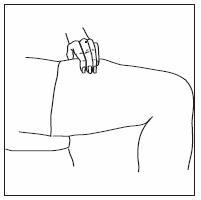
If home use is determined to be desirable by the physician, instructions on appropriate use should be given by a healthcare professional. After administration of INFERGEN, it is essential to follow the procedure for proper disposal of syringes and needles. [see Medication Guide for detailed instructions].
3. Dosage Forms and Strengths
INFERGEN is provided in single-use vials containing:
- •
- 9 mcg/0.3 mL INFERGEN in sterile, clear, colorless, preservative-free liquid
- •
- 15 mcg/0.5 mL INFERGEN in sterile, clear, colorless, preservative-free liquid
4. Contraindications
INFERGEN is contraindicated in patients with
- •
- hepatic decompensation (Child-Pugh score >6 [class B and C])
- •
- autoimmune hepatitis
- •
- known hypersensitivity reactions such as urticaria, angioedema, bronchoconstriction, anaphylaxis to interferon alphas or to any component of the product
Additionally, ribavirin is contraindicated in:
- •
- women who are pregnant
- •
- men whose female partners are pregnant
- •
- patients with hemoglobinopathies (e.g., thalassemia major, sickle-cell anemia)
- •
- patients with hypersensitivity to ribavirin or any other component of the product
- •
- patients with creatinine clearance <50 mL/min
5. Warnings and Precautions
Treatment with INFERGEN and combination treatment with INFERGEN/ribavirin should be administered under the guidance of a qualified physician, and may lead to moderate-to-severe adverse reactions requiring dose reduction, temporary dose cessation, or discontinuation of further therapy.
5.1 Use with Ribavirin
Pregnancy
Ribavirin may cause birth defects and death of the unborn child. Ribavirin therapy should not be started until a report of a negative pregnancy test has been obtained immediately prior to planned initiation of therapy. Patients should use at least two forms of contraception and have monthly pregnancy tests. Pregnancy should be avoided for at least six months after discontinuation of ribavirin [see BOXED WARNING, CONTRAINDICATIONS (4), Use in Specific Populations (8.1), Patient Counseling Information (17) and Ribavirin Labeling].
Anemia
Ribavirin caused hemolytic anemia in 30% of INFERGEN/ribavirin-treated subjects. Complete blood counts should be obtained pretreatment and at Week 2 and Week 4 of therapy or more frequently if clinically indicated. Anemia associated with ribavirin therapy may result in a worsening of cardiac disease. Decrease in dosage or discontinuation of ribavirin may be necessary [see Dosage and Administration (2.3) and Ribavirin Labeling].
5.2 Neuropsychiatric Disorders
Severe psychiatric adverse reactions may manifest in patients receiving therapy with interferon alphas, including INFERGEN. Depression, suicidal ideation, suicide attempt, suicide, and homicidal ideation may occur. Other prominent psychiatric adverse reactions including psychosis, aggressive behavior, nervousness, anxiety, emotional lability, abnormal thinking, agitation, apathy and relapse of drug addiction may occur. INFERGEN should be used with extreme caution in patients who report a history of depression. Physicians should monitor all patients for evidence of depression and other psychiatric symptoms. Prior to initiation of INFERGEN therapy, physicians should inform patients of the possible development of depression and patients should be advised to report any sign or symptom of depression and/or suicidal ideation immediately. If patients develop psychiatric problems, including clinical depression, it is recommended that the patients be carefully monitored during treatment and in the 6-month follow-up period. If psychiatric symptoms persist or worsen, or suicidal ideation or aggressive behavior towards others are identified, it is recommended that treatment with INFERGEN be discontinued, and the patient followed, with psychiatric intervention as appropriate. In severe cases, INFERGEN should be stopped immediately and psychiatric intervention instituted [see DOSAGE AND ADMINISTRATION: Dose Modifications (2.3)].
5.3 Cardiovascular Events
Cardiovascular events, which include hypotension, arrhythmia, tachycardia, cardiomyopathy, angina pectoris, and myocardial infarction, have been observed in patients treated with INFERGEN. INFERGEN should be used cautiously in patients with cardiovascular disease. Patients with a history of myocardial infarction and arrhythmic disorder who require INFERGEN therapy should be closely monitored [see WARNINGS and PRECAUTIONS (5)]. Patients with a history of significant or unstable cardiac disease should not be treated with INFERGEN/ribavirin combination therapy [see Ribavirin Labeling].
5.4 Pulmonary Disorders
Dyspnea, pulmonary infiltrates, pneumonia, bronchiolitis obliterans, interstitial pneumonitis, pulmonary hypertension and sarcoidosis, some resulting in respiratory failure and/or patient deaths, may be induced or aggravated by interferon alpha therapy, including INFERGEN. Patients who develop persistent or unexplained pulmonary infiltrates or pulmonary function impairment should discontinue treatment with INFERGEN. Recurrence of respiratory failure has been observed with interferon rechallenge. INFERGEN treatment should be suspended in patients who develop pulmonary infiltrates or pulmonary function impairment. Patients who resume interferon treatment should be closely monitored.
5.5 Hepatic Failure
Chronic hepatitis C patients with cirrhosis may be at risk of hepatic decompensation when treated with interferon alphas, including INFERGEN. During treatment, patients’ clinical status and hepatic function should be closely monitored, and INFERGEN treatment should be immediately discontinued if symptoms of hepatic decompensation, such as jaundice, ascites, coagulopathy, or decreased serum albumin are observed [see CONTRAINDICATIONS (4)].
5.6 Renal Insufficiency
Increases in serum creatinine levels, including renal failure, have been observed in patients receiving INFERGEN. INFERGEN has not been studied in patients with renal insufficiency. It is recommended that renal function be evaluated in all patients starting INFERGEN alone or with ribavirin therapy. Patients with impaired renal function should be closely monitored for signs and symptoms of interferon toxicity, including increases in serum creatinine. Combination treatment with INFERGEN/ribavirin should not be used in patients with creatinine clearance <50 mL/min. [see CONTRAINDICATIONS (4) and Ribavirin Labeling].
5.7 Cerebrovascular Disorders
Ischemic and hemorrhagic cerebrovascular events have been observed in patients treated with interferon alpha-based therapies, including INFERGEN. Events occurred in patients with few or no reported risk factors for stroke, including patients less than 45 years of age. Because these are spontaneous reports, estimates of frequency cannot be made and a causal relationship between interferon alpha-based therapies and these events is difficult to establish.
5.8 Bone Marrow Toxicity
Interferon alphas suppress bone marrow function and may result in severe cytopenias including aplastic anemia. It is advised that complete blood counts be obtained pretreatment and monitored routinely during therapy. INFERGEN therapy should be discontinued in patients who develop severe decreases in neutrophil (< 0.5 x 109/L) or platelet counts (< 25 x 109/L).
INFERGEN should be used cautiously in patients with abnormally low peripheral blood cell counts or who are receiving agents that are known to cause myelosuppression. Transplantation patients or other chronically immunosuppressed patients should be treated with interferon alpha therapy with caution.
The use of ribavirin may result in a worsening of INFERGEN-induced neutropenia. Therefore combination treatment with INFERGEN/ribavirin should be used with caution in patients with low baseline neutrophil counts (< 1500 cells/mm3) and may require that therapy be discontinued in the event of a severe decrease in neutrophil count [see DOSAGE AND ADMINISTRATION: Dose Modifications (2.3) and WARNINGS AND PRECAUTIONS: Laboratory Tests (5.16)].
5.9 Colitis
Hemorrhagic/ischemic colitis, sometimes fatal, has been observed within 12 weeks of interferon alpha therapies and has been reported in patients treated with INFERGEN. INFERGEN treatment should be discontinued immediately in patients who develop signs and symptoms of colitis.
5.10 Pancreatitis
Pancreatitis, sometimes fatal, has been observed in patients treated with interferon alphas, including INFERGEN. INFERGEN should be suspended in patients with signs and symptoms suggestive of pancreatitis and discontinued in patients diagnosed with pancreatitis.
5.11 Hypersensitivity
Serious acute hypersensitivity reactions have been reported following treatment with interferon alphas. If hypersensitivity reactions occur (e.g., urticaria, angioedema, bronchoconstriction, anaphylaxis), INFERGEN should be discontinued immediately and appropriate medical treatment instituted.
5.12 Autoimmune Disorders
Development or exacerbation of autoimmune disorders (e.g., autoimmune thrombocytopenia, idiopathic thrombocytopenic purpura, psoriasis, rheumatoid arthritis, thyroiditis, interstitial nephritis, systemic lupus erythematosus (SLE)) have been reported in patients receiving interferon alpha therapies, including INFERGEN. INFERGEN should not be used in patients with autoimmune hepatitis [see CONTRAINDICATIONS (4)] and should be used with caution in patients with other autoimmune disorders.
5.13 Ophthalmologic Disorders
Decrease or loss of vision, retinopathy including macular edema, retinal artery or vein thrombosis, retinal hemorrhages and cotton wool spots; optic neuritis, papilledema, and serous retinal detachment are induced or aggravated by treatment with INFERGEN or other interferons alpha. All patients should receive an eye examination at baseline. Patients with preexisting ophthalmologic disorders (e.g., diabetic or hypertensive retinopathy) should receive periodic ophthalmologic exams during interferon alpha treatment. Any patient who develops ocular symptoms should receive a prompt and complete eye examination. INFERGEN therapy should be discontinued in patients who develop new or worsening ophthalmologic disorders.
5.14 Peripheral Neuropathy
Peripheral neuropathy has been reported when interferon alphas were given in combination with telbivudine. In one clinical trial, an increased risk and severity of peripheral neuropathy was observed with the combination use of telbivudine and pegylated interferon alfa-2a as compared to telbivudine alone. The safety and efficacy of telbivudine in combination with interferons for the treatment of chronic hepatitis B has not been demonstrated.
5.15 Endocrine Disorders
INFERGEN should be administered with caution to patients with a history of endocrine disorders. Occurrence or aggravation of hyperthyroidism or hypothyroidism have been reported with INFERGEN. Hyperglycemia and diabetes mellitus have also been observed in patients treated with INFERGEN. Patients who develop these conditions during treatment that cannot be controlled with medication should not continue INFERGEN therapy.
5.16 Laboratory Tests
Laboratory tests are recommended for all patients on INFERGEN therapy, as follows: prior to beginning treatment (baseline), 2 weeks after initiation of therapy, and periodically thereafter during the 24 or 48 weeks of therapy at the discretion of the physician. Following completion of INFERGEN therapy, any abnormal test values should be monitored periodically. The entrance criteria that were used for the clinical study of INFERGEN may be considered as a guideline to acceptable baseline values for initiation of treatment:
- •
- Platelet count ≥ 75 × 109/L
- •
- Hemoglobin concentration ≥ 10 g/dL
- •
- ANC ≥ 1500 × 106/L
- •
- Serum creatinine concentration < 180 µmol/L (< 2.0 mg/dL) or creatinine clearance > 0.83 mL/second (> 50 mL/minute)
- •
- Serum albumin concentration ≥ 25 g/L
- •
- Bilirubin ≤ 1.4 mg/dL (with the exception of patients with Gilbert’s syndrome)
- •
- TSH and T4 within normal limits
Neutropenia, thrombocytopenia, hypertriglyceridemia and thyroid disorders have been reported with administration of INFERGEN [see ADVERSE REACTIONS]. Therefore, these laboratory parameters should be monitored closely.
Patients who have pre-existing cardiac abnormalities should have electrocardiograms administered before treatment with INFERGEN/ribavirin.
6. Adverse Reactions/Side Effects
INFERGEN alone or in combination with ribavirin causes a broad range of serious adverse reactions [see BOXED WARNING and WARNINGS AND PRECAUTIONS (5)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical development, more than 560 subjects were exposed to 9 mcg or 15 mcg of INFERGEN monotherapy administered three times per week over a range of 24 to 48 weeks, and more than 480 subjects were exposed to 9 mcg or 15 mcg of INFERGEN, in combination with ribavirin, administered daily up to 48 weeks.
INFERGEN Monotherapy Clinical Trials
Adverse reactions that were reported, regardless of attribution to treatment, in ≥ 10% of subjects in INFERGEN monotherapy studies are presented in Table 4.
Flu-like symptoms (i.e., headache, fatigue, fever, rigors, myalgia, arthralgia, and sweating increased) were the most frequently reported treatment-related adverse reactions. In most cases, these events could be treated symptomatically.
Depression of any severity was reported in 26% of subjects who received 9 mcg INFERGEN monotherapy and was the most common adverse reaction resulting in study drug discontinuation.
INFERGEN 15 mcg three times a week monotherapy as subsequent treatment was associated with a greater incidence of leukopenia and granulocytopenia. One or more dose reductions for any causes were required in up to 36% of subjects.
|
Initial Treatment |
Subsequent Treatment |
|||
|
INFERGEN |
IFN α-2b |
INFERGEN |
INFERGEN |
|
|
Body System/Preferred Term (COSTART) |
% of Subjects |
% of Subjects |
||
|
APPLICATION SITE | ||||
|
Injection Site Erythema |
23 |
15 |
17 |
22 |
|
BODY AS A WHOLE | ||||
|
Fatigue |
69 |
67 |
65 |
71 |
|
Fever |
61 |
45 |
58 |
55 |
|
Rigors |
57 |
45 |
62 |
66 |
|
Body Pain |
54 |
45 |
39 |
51 |
|
Influenza-like Symptoms |
15 |
11 |
8 |
8 |
|
Chest Pain |
13 |
14 |
5 |
9 |
|
Hot Flushes |
13 |
7 |
7 |
4 |
|
Malaise |
11 |
10 |
2 |
5 |
|
Asthenia |
9 |
11 |
10 |
7 |
|
CNS/PNS | ||||
|
Headache |
82 |
83 |
78 |
80 |
|
Insomnia |
39 |
30 |
24 |
28 |
|
Dizziness |
22 |
25 |
18 |
25 |
|
Paresthesia |
13 |
10 |
9 |
9 |
|
Hypoesthesia |
10 |
8 |
8 |
10 |
|
Amnesia |
10 |
6 |
2 |
5 |
|
GASTROINTESTINAL | ||||
|
Abdominal Pain |
41 |
40 |
24 |
32 |
|
Nausea |
40 |
36 |
30 |
36 |
|
Diarrhea |
29 |
24 |
24 |
22 |
|
Anorexia |
24 |
17 |
21 |
14 |
|
Dyspepsia |
21 |
18 |
12 |
10 |
|
Vomiting |
12 |
11 |
13 |
11 |
|
MUSCULO-SKELETAL | ||||
|
Myalgia |
58 |
56 |
51 |
55 |
|
Arthralgia |
51 |
44 |
43 |
46 |
|
Back Pain |
42 |
37 |
29 |
23 |
|
Limb Pain |
26 |
25 |
13 |
23 |
|
Skeletal Pain |
14 |
14 |
10 |
12 |
|
Neck Pain |
14 |
13 |
8 |
5 |
|
PSYCHIATRIC DISORDER | ||||
|
Nervousness |
31 |
29 |
16 |
22 |
|
Depression |
26 |
25 |
18 |
19 |
|
Anxiety |
19 |
18 |
9 |
14 |
|
Emotional Lability |
12 |
11 |
6 |
3 |
|
Thinking Abnormal |
8 |
12 |
10 |
20 |
|
RESPIRATORY | ||||
|
Pharyngitis |
34 |
31 |
17 |
21 |
|
Cough |
22 |
17 |
12 |
11 |
|
Sinusitis |
17 |
22 |
12 |
16 |
|
Dyspnea |
7 |
12 |
8 |
7 |
|
SKIN AND APPENDAGES | ||||
|
Alopecia |
14 |
25 |
10 |
13 |
|
Pruritus |
14 |
14 |
11 |
10 |
|
Rash |
13 |
15 |
13 |
10 |
|
Sweating Increased |
12 |
11 |
13 |
11 |
Combination Treatment with INFERGEN/Ribavirin Clinical Trials
The most common adverse reactions in the combination treatment with INFERGEN/ribavirin trial are listed in Table 5 and included fatigue (76%), nausea (45%), flu-like symptoms (40%), headache (42%), arthralgia (31%), and myalgia (29%), neutropenia (40%), leukopenia (29%), insomnia (39%), and depression (26%).
Adverse reactions led to early study discontinuation in 104 (21%) of subjects; more subjects discontinued from the 15 mcg INFERGEN group (64 versus 40). Fatigue, anemia, and depression were the most common adverse reactions resulting in study drug discontinuation. A higher proportion of subjects who received the recommended starting dose of 15 mcg (52%) than the 9 mcg dose group (40%) required INFERGEN dose modifications due to adverse reactions, primarily due to neutropenia/leukopenia, thrombocytopenia, and fatigue/weakness. A total of 14% of subjects experienced serious adverse reactions, the most common of which were neutropenia (2%), suicidal ideation (1%), and hyperuricemia (1%).
|
Retreatment |
||
|
INFERGEN 9 mcg/RBV 48 wks (n = 244) |
INFERGEN 15 mcg/RBV 48 wks (n = 242) |
|
|
Body System/Preferred Term (MedDRA) |
% of Subjects |
|
|
GASTROINTESTINAL DISORDERS |
||
|
Abdominal pain |
15 |
14 |
|
Constipation |
9 |
10 |
|
Diarrhea |
18 |
19 |
|
Nausea |
45 |
45 |
|
Vomiting |
12 |
19 |
|
GENERAL DISORDERS and ADMINISTRATION SITE CONDITIONS (or BODY AS A WHOLE) |
||
|
Fatigue |
75 |
77 |
|
Influenza-like Illness (or Symptoms) |
40 |
42 |
|
Injection Site Erythema |
16 |
16 |
|
Injection Site Reaction |
15 |
12 |
|
Pyrexia (or Fever) |
13 |
17 |
|
Rigors |
19 |
22 |
|
INVESTIGATIONS | ||
|
Weight Decrease |
16 |
22 |
|
METABOLISM and NUTRITION DISORDERS |
||
|
Anorexia |
15 |
21 |
|
Decreased appetite |
17 |
18 |
|
MUSCULOSKELETAL and CONNECTIVE TISSUE DISORDERS |
||
|
Arthralgia |
31 |
31 |
|
Back Pain |
12 |
9 |
|
Myalgia |
24 |
34 |
|
NERVOUS SYSTEM DISORDERS |
||
|
Dizziness |
14 |
19 |
|
Headache |
46 |
39 |
|
PSYCHIATRIC DISORDER |
||
|
Anxiety |
12 |
11 |
|
Depression |
27 |
25 |
|
Insomnia |
39 |
38 |
|
Irritability |
21 |
17 |
|
RESPIRATORY, THORACIC, and MEDIASTINAL DISORDERS |
||
|
Cough |
14 |
17 |
|
Dyspnea |
15 |
20 |
|
SKIN and SUBCUTANEOUS TISSUE DISORDERS |
||
|
Alopecia |
10 |
10 |
|
Pruritus |
15 |
11 |
|
Rash |
17 |
12 |
Laboratory Values
Hemoglobin and Hematocrit: Treatment with INFERGEN alone and in combination with ribavirin is associated with decreases in mean values for hemoglobin and hematocrit. In the INFERGEN monotherapy trials, 4% and 5% of subjects had decreases in hemoglobin and hematocrit levels. Decreases from baseline of 20% or more in hemoglobin or hematocrit were seen in ≤1% of subjects.
In the combination INFERGEN/ribavirin trial, 88% of subjects had decreases in hemoglobin levels of ≥2 g/dL from baseline. Of these, 27% had hemoglobin levels decrease to ≤10 g/dL, and underwent dose reductions of ribavirin. Anemia or hemolytic anemia led to study drug discontinuation in 10 subjects.
White Blood Cells: INFERGEN treatment is associated with decreases in mean values for both total white blood cell (WBC) count and ANC. By the end of initial monotherapy treatment, mean decreases from baseline of 19% for WBCs and 23% for ANC were observed. These effects reversed during the post treatment observation period. In two INFERGEN-monotherapy treated subjects ANC levels decreased to below 500 × 106 cells/L. In both cases, the ANC values returned to clinically acceptable levels with INFERGEN dose reductions and were not associated with infections.
Mean decreases from baseline up to 23% for WBCs and up to 27% for ANC were observed for subjects subsequently retreated with INFERGEN monotherapy. Two subjects experienced reversible reductions in ANC to less than 500 × 106 cells/L.
In the combination INFERGEN/ribavirin trial, leukopenia was reported in 24% and 34% of 9 mcg and 15 mcg treated subjects, respectively. More subjects treated with 15 mcg experienced lymphopenia than did those treated with 9 mcg: 14% versus 7%. ANC levels <0.75 x 109/L were observed in 21% of subjects treated with 9 mcg and 27% of those treated with 15 mcg; no subjects experienced significant infections associated with low ANC levels.
Platelets: INFERGEN treatment is associated with alterations in platelet count. Decreases in mean platelet count of 16% compared to baseline were seen by the end of INFERGEN monotherapy treatment. These decreases were reversed during the post treatment observation period. Three percent of subjects had platelets decrease to less than 50 × 109 cells/L, which necessitated dose reduction.
More subjects treated with 15 mcg in the INFERGEN/ribavirin combination trial experienced a decrease in platelet counts <40 × 109/L, 3% versus 1% in the 9 mcg dose group. None of the subjects had platelet counts <25 × 109/L. One subject in the 15 mcg group had Grade 4 thrombocytopenia 127 days after the start of treatment, was hospitalized for this event, and treatment with both study drugs was discontinued; the event resolved 8 days later.
Triglycerides: Mean values for serum triglyceride increased shortly after the start of administration of INFERGEN monotherapy, with increases of 41%, compared with baseline, at the end of the treatment period. Seven percent of the subjects developed values which were at least 3 times above pretreatment levels during treatment. This effect was reversed after discontinuation of treatment.
In the INFERGEN/ribavirin combination trial, 7% of subjects in the 15 mcg dose group experienced increases in triglyceride levels over baseline levels at week 48 compared to 2% in the 9 mcg dose group. There were no differences in the proportion of subjects who had ≥Grade 3 triglyceride elevations: 2% in both dose groups.
Thyroid Function: INFERGEN monotherapy treatment was associated with biochemical changes consistent with hypothyroidism including increases in TSH and decreases in T4 mean values. Increases in TSH to greater than 7 mU/L were seen in 10% of 9 mcg INFERGEN-treated subjects either during the treatment period or the 24-week post treatment observation period. Thyroid supplements were instituted in approximately one-third of these subjects.
In the combination INFERGEN/Ribavirin trial, mean increases in TSH levels from baseline were greater for the 15 mcg group compared with the 9 mcg group; 14% and 3%, respectively, at Week 12 and 54% and 0% at Week 48. No serious adverse events, discontinuations or dose modifications were related to abnormalities in thyroid function.
Uric Acid: Grade 4 (>10 mg/dL) uric acid levels were commonly observed in both INFERGEN/ribavirin treatment groups: 23 in the 9 mcg and 26 in the 15 mcg group. One subject in the 9 mcg group and three in the 15 mcg group experienced serious adverse events related to elevated uric acid levels. Four subjects in the 15 mcg had INFERGEN/ribavirin temporarily interrupted due to elevated uric acid levels.
6.2 Immunogenicity
The number of subjects developing positive binding antibody responses was similar in the 9 mcg INFERGEN (11%) and 3 MIU IFN α-2b groups (15%) in monotherapy studies. The titer of neutralizing antibodies to interferon was not measured. Following cessation of interferon therapy, the number of subjects with a positive antibody response declined.
In the INFERGEN/ribavirin combination study, approximately 13% of subjects in the 15 mcg and 18% in the 9 mcg arms developed low-titer neutralizing antibodies to INFERGEN. The clinical and pathological significance of the appearance of serum neutralizing antibodies is unknown. No apparent correlation of antibody development to clinical response was observed. The incidence of binding antibody was approximately 31%.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies for INFERGEN with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified and reported during post-approval use of INFERGEN. Because these reactions are reported voluntarily and from a population of uncertain size, it is not possible to reliably estimate the frequency of the reaction or establish a causal relationship to drug exposure.
Application site
injection site reaction, including injection site necrosis ulcer, and bruising
Ear and Labyrinth
hearing loss, hearing impairment
Gastrointestinal
abdominal distention, gastrointestinal bleeding, gastritis
Hepatobiliary
hepatic enzyme elevations, including ALT and AST elevation, abnormal hepatic function, hyperbilirubinemia, jaundice, ascites, hepatic encephalopathy
Infections
sepsis
Metabolism and Nutritional
dehydration
Musculoskeletal
rhabdomyolysis, arthritis, bone pain
Nervous
speech disorder, ataxia, gait abnormal, convulsions, loss of consciousness, memory impairment, tremors, visual field defect
Psychiatric
delusions, hallucinations
Skin and Subcutaneous
bruising, pyoderma gangrenosum, toxic epidermal necrolysis
Vascular Disorders
Hemorrhage
Related/similar drugs
Epclusa
Epclusa treats chronic hepatitis C in adults and children 3+. This once-daily antiviral combines ...
Mavyret
Mavyret is used to treat adults and children 3 years of age and older with acute or chronic ...
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Harvoni
Harvoni is used to treat hepatitis C virus (HCV) infections in adults and children aged 3 years and ...
Sovaldi
Sovaldi (sofosbuvir) is used to treat chronic hepatitis C virus (HCV) infection. Includes Sovaldi ...
Pegasys
Pegasys is used to treat chronic hepatitis B or C. Learn about side effects, interactions and ...
Vosevi
Vosevi (sofosbuvir,velpatasvir and voxilaprevir) is used to treat chronic hepatitis C. Includes ...
Interferon alfa-2b
Interferon alfa-2b is used for angioblastoma, condylomata acuminata, conjunctival mucosa-associated ...
7. Drug Interactions
8. Use In Specific Populations
8.1 Pregnancy
INFERGEN Monotherapy
Pregnancy Category C
INFERGEN has been shown to have embryo lethal or abortifacient effects in golden Syrian hamsters when given at doses > 150 mcg/kg/day (135 times the human dose) and in cynomolgus and rhesus monkeys when given at doses of 3 mcg/kg/day and 10 mcg/kg/day (9 to 81 times the human dose), respectively, based on body surface area, the human dose. There are no adequate and well-controlled studies in pregnant women. INFERGEN should not be used during pregnancy. If a woman becomes pregnant or plans to become pregnant while taking INFERGEN, she should be informed of the potential hazards to the fetus. Males and females treated with INFERGEN should be advised to use effective contraception.
Combination Treatment with INFERGEN/Ribavirin
Pregnancy Category X
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. Ribavirin therapy is contraindicated in women who are pregnant and in the male partners of women who are pregnant [see CONTRAINDICATIONS (4) and Ribavirin Labeling].
Ribavirin Pregnancy Registry: A Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Physicians and patients are encouraged to report such cases by calling 1-800-593-2214.
8.3 Nursing Mothers
It is not known whether INFERGEN or ribavirin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if INFERGEN is administered to a nursing woman. The effect on the nursing neonate of orally ingested INFERGEN in breast milk has not been evaluated. Because of the potential for serious adverse reactions from the drug in nursing infants, a decision should be made whether to discontinue nursing or to delay or discontinue ribavirin.
8.4 Pediatric Use
The safety and effectiveness of INFERGEN have not been established in patients below the age of 18 years. INFERGEN therapy is not recommended in pediatric patients.
8.5 Geriatric Use
Clinical studies of INFERGEN alone or in combination with ribavirin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, treatment with interferons, including INFERGEN, is associated with psychiatric, cardiac, and systemic (flu-like) adverse reactions. Since decreased hepatic, renal or cardiac function, concomitant disease, and the use of other drug therapies in elderly patients may produce adverse reactions of greater severity, caution should be exercised in the use of INFERGEN and INFERGEN/ribavirin in this population. Ribavirin should not be used in patients with creatinine clearance <50 mL/min.
8.6 Hepatic Impairment
The safety and efficacy of INFERGEN, alone or in combination with ribavirin, for the treatment of chronic HCV infection in patients with hepatic impairment has not been studied. The use of INFERGEN in patients with hepatic decompensation (Child-Pugh score >6 [class B and C]) is contraindicated [see CONTRAINDICATIONS (4)].
8.7 Renal Impairment
The safety and efficacy of INFERGEN, alone or in combination with ribavirin, for the treatment of chronic HCV infection in patients with renal impairment has not been studied. In patients with impaired renal function, signs and symptoms of interferon toxicity should be closely monitored and INFERGEN dose should be adjusted as recommended in Tables 1-3.
INFERGEN/ribavirin should not be administered to patients with creatinine clearance <50 mL/min [see DOSAGE AND ADMINISTRATION: Dose Modifications (2.3), CONTRAINDICATIONS (4) and Ribavirin Labeling].
10. Overdosage
In INFERGEN trials, the maximum overdose reported was a dose of 150 mcg INFERGEN administered subcutaneously in a subject enrolled in a phase 1 advanced malignancy trial. The subject received 10 times the prescribed dosage for three days and experienced a mild increase in anorexia, chills, fever, and myalgia. Increases in ALT (15 IU/L to 127 IU/L), aspartate transaminase (AST) (15 to 164 IU/L), and lactic dehydrogenase (LDH) (183 IU/L to 281 IU/L) were reported. These laboratory values returned to normal or to the subjects baseline values within 30 days.
11. Infergen Description
Interferon alfacon-1 is a wholly synthetic type-I interferon. The 166-amino acid sequence of interferon alfacon-1 was derived by scanning the sequences of several natural interferon alpha subtypes and assigning the most frequently observed amino acid in each corresponding position resulting in a consensus sequence. Four additional amino acid changes were made to facilitate the molecular construction, and a corresponding synthetic DNA sequence was constructed using chemical synthesis methodology. Interferon alfacon-1 differs from interferon alfa-2b at 19/166 amino acids (88% homology), and with Interferon alfa-2a at 18/166 amino acids (88% homology). Comparison with interferon-beta shows identity at over 30% of the amino acid positions. Interferon alfacon-1 is produced in Escherichia coli (E. coli) cells that have been genetically altered by insertion of a synthetically constructed sequence that codes for interferon alfacon-1. Prior to final purification, interferon alfacon-1 is allowed to oxidize to its native state, and its final purity is achieved by sequential passage over a series of chromatography columns. This protein has a molecular weight of 19,434 daltons.
INFERGEN is a sterile, clear, colorless, preservative-free liquid formulated with 100 mM sodium chloride and 27 mM sodium phosphate at pH 7.0 ± 0.2. The product is available in single-use vials containing 9 mcg and 15 mcg interferon alfacon-1 at a fill volume of 0.3 mL and 0.5 mL, respectively. INFERGEN vials contain 0.03 mg/mL interferon alfacon-1, sodium chloride (5.9 mg/mL), and sodium phosphate (3.8 mg/mL) in Water for Injection, USP. INFERGEN is to be administered undiluted by subcutaneous injection.
12. Infergen - Clinical Pharmacology
12.1 Mechanism of Action
Interferon alfacon-1 is an inducer of the innate antiviral immune response. [see CLINICAL PHARMACOLOGY (12.4)].
12.2 Pharmacodynamics
Interferons induce pleiotropic biologic responses which include antiviral, antiproliferative, and immunomodulatory effects, regulation of cell surface major histocompatibility antigen (HLA class I and class II) expression and regulation of cytokine expression.
Analysis of INFERGEN-induced cellular products (induction of 2'5' OAS and ß-2 microglobulin) after treatment in these subjects revealed a statistically significant, dose-related increase in the area under the curve (AUC) for the levels of 2'5' OAS or ß-2 microglobulin induced over time. Concentrations of 2'5' OAS were maximal at 24 hours after dosing, while serum levels of ß-2 microglobulin appeared to reach a maximum 24 to 36 hours after dosing. The dose-response relationships observed for 2'5' OAS and ß-2 microglobulin were indicative of biological activity after subcutaneous injection administration of 1 mcg to 9 mcg INFERGEN.
12.3 Pharmacokinetics
The pharmacokinetic properties of INFERGEN have not been evaluated in patients with chronic hepatitis C. Pharmacokinetic profiles were evaluated in normal, healthy volunteer subjects after subcutaneous injection of 1 mcg, 3 mcg, or 9 mcg INFERGEN. Plasma levels of INFERGEN after subcutaneous injection administration of any dose were too low to be detected by either enzyme-linked immunosorbent assay (ELISA) or by inhibition of viral cytopathic effect.
Renal Dysfunction
Patients with creatinine clearance <50 mL/min should not be treated with ribavirin [see WARNINGS AND PRECAUTIONS: Renal Impairment (5.6); Ribavirin Labeling].
12.4 Microbiology
Mechanism of Action
Interferon alfacon-1 is a recombinant hybrid protein based on the consensus amino acid sequence of naturally occurring human type-I interferon alphas. Type-I interferons are a family of small protein molecules with molecular weights of 15,000 to 21,000 daltons that are produced and secreted by cells in response to viral infections or to various synthetic and biological inducers. Interferons do not act directly on the virus but bind to the interferon cell-surface receptor leading to the production of several interferon-stimulated gene products. Interferons induce pleiotropic biologic responses which include antiviral, antiproliferative, and immunomodulatory effects, regulation of cell surface major histocompatibility antigen (HLA class I and class II) expression and regulation of cytokine expression.
Antiviral Activity in Cell Culture
The antiviral activity of INFERGEN, alone or in combination with ribavirin, against HCV or HCV-derived replicons in cell culture has not been determined.
Resistance
HCV genotypes show wide variability in their response to interferon/ribavirin based therapies. Genetic changes associated with the variable response have not been identified. It has been reported that certain regions of the HCV genome, especially a region in the NS5B protein called IFN-sensitive determining region, may play a role in determination of a patient’s response to interferon treatment.
Cross-resistance
The homology between interferon alfacon-1 and other type-I interferons, and the clinical responses for the different HCV genotypes are consistent with cross-resistance.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: No carcinogenicity data for INFERGEN are available in animals or humans.
Mutagenesis: INFERGEN was not mutagenic when tested in several in vitro assays, including the Ames bacterial mutagenicity assay and an in vitro cytogenetic assay in human lymphocytes, either in the presence or absence of metabolic activation.
Use with Ribavirin: See ribavirin labeling for additional warnings relevant to INFERGEN therapy in combination with ribavirin.
Impairment of Fertility: INFERGEN at doses as high as 100 mcg/kg did not selectively affect reproductive performance or the development of the offspring when administered subcutaneous injection to male and female golden Syrian hamsters for 70 and 14 days before mating, respectively, and then through mating and to day 7 of pregnancy.
13.2 Animal Toxicology and/or Pharmacology
Animal Toxicology
In preclinical toxicology studies in golden Syrian hamsters and rhesus monkeys, administration of INFERGEN at doses of up to 100 mcg/kg/day was associated with decreased body weight, decreased food consumption, and bone marrow suppression. High-dose chronic exposure at doses of 10 mcg/kg/day to 100 mcg/kg/day (50-fold to 500-fold higher than the maximum clinical dose given daily) in rhesus monkeys was not tolerated for greater than 1 month, due to the development of vascular leak syndrome.
14. Clinical Studies
14.1 Initial Treatment with INFERGEN Monotherapy
The efficacy of INFERGEN monotherapy compared to recombinant human interferon alfa-2b (IFN α-2b) was evaluated in a randomized, double-blind clinical trial involving 704 subjects previously untreated with interferon alpha. Subjects were 18 years or older, had compensated liver disease, tested positive for HCV RNA, and had elevated serum alanine aminotransferase (ALT) averaging greater than 1.5 times the upper limit of normal. Staging of chronic liver disease was confirmed by a liver biopsy taken within 1 year prior to enrollment.
Subjects were treated with INFERGEN 3 mcg (n = 232), 9 mcg (n = 232), or IFN α-2b 3 million international units (MIU) (n = 240), each administered three times per week for 24 weeks and were observed for 24 weeks after the end of treatment. Efficacy was determined by measurement of serum ALT and HCV RNA levels, and changes in liver histology. Serum HCV RNA was assessed using a research-based quantitative reverse transcriptase polymerase chain reaction (RT-PCR) assay with a lower limit of sensitivity of 100 copies/mL. Liver histology was assessed by comparing the histology activity index (HAI) score of pretreatment and post treatment biopsy specimens. Histologic improvement was defined as having at least a 2-unit decrease in the Knodell HAI score.
Response rates at the end of the observation period are included in Table 6.
| a 3 MIU IFN α-2b is equivalent to approximately 15 mcg IFN α-2b. | ||
|
INFERGEN
|
IFN α-2b
|
|
|
Normalized ALT |
17% |
17% |
|
HCV RNA negative |
9% |
8% |
|
Histologic improvement |
68% |
65% |
The 3 mcg INFERGEN dosage arm was substantially less effective with only 3% of subjects achieving end of observation responses.
14.2 Subsequent Treatment with INFERGEN Monotherapy
Subsequent treatment with INFERGEN 15 mcg monotherapy for either 24 or 48 weeks was evaluated in an open-label clinical trial of 208 subjects who had failed initial interferon monotherapy. Of the subjects, 64% had failed to normalize ALT during initial treatment (ALT non-responder) and 36% achieved normal ALT levels during initial treatment, but had return of elevated ALT levels during post treatment observation (ALT relapse). Subjects were assessed for normalization of ALT and HCV RNA reduction to ≤ 100 copies/mL at the end of 24 weeks of observation following discontinuation of therapy. Response rates are included in Table 7.
|
All Subjects |
Prior ALT Nonresponders |
Prior ALT Relapsers |
|||
|
24 Weeks
|
48 Weeks
|
24 Weeks
|
48 Weeks
|
24 Weeks
|
48 Weeks
|
|
Normalized ALT |
|||||
|
13% |
19% |
7% |
7% |
27% |
36% |
|
HCV RNA <100 copies/mL |
|||||
|
9% |
22% |
4% |
12% |
21% |
36% |
14.3 Subsequent Treatment with Combination INFERGEN/Ribavirin
This study (DIRECT Trial/ IRHC-001 and IRHC-002) was a randomized, open-label, multi-center, US-based study comparing the safety and efficacy of two doses of INFERGEN (9 mcg or 15 mcg) administered daily plus ribavirin (1000 mg or 1200 mg weight based dosed) administered daily for 48 weeks to subjects who were nonresponders to previous pegylated interferon plus ribavirin (Peg-IFN/ribavirin) therapy. Prior non-response was defined as a < 2 log10 decline in viral load (VL) while undergoing at least 12 weeks of previous Peg-IFN/ribavirin therapy with ≥ 80% adherence or a detectable VL at end-of-treatment after completing at least 24 weeks of therapy. Study subjects had a mean age of 50 yrs, 70% were male, mean weight of 89 kg, 19% were African Americans, 65% were Caucasians, 66% had high VL (≥ 850,000 IU/mL), 95% were infected with genotype 1, 54% had evidence of bridging fibrosis, 25% had evidence of cirrhosis on biopsy, and 50% had steatosis. Approximately, 80% of the subjects were null responders (< 2 log10 drop in viral load during their previous Peg-IFN/ribavirin therapy). The median washout period between previous treatment and day 1 of INFERGEN therapy was 448 days (15 months) and 506 days (16.8 months) for the 9 mcg and 15 mcg groups, respectively. The use of hematopoietic growth factors was not permitted in the DIRECT Trial.
In study IRHC-001, 515 subjects were randomized to INFERGEN 9 mcg plus ribavirin (n=171), INFERGEN 15 mcg plus ribavirin (n=172), or no treatment (n=172). In study IRHC-002, 144 subjects in the no treatment arm of study IRHC-001 were re-randomized to either INFERGEN 9 mcg plus ribavirin (n=74) or INFERGEN 15 mcg plus ribavirin (n=70).
Subjects were treated for up to 48 weeks. The primary endpoint was sustained virological response (SVR), defined as undetectable HCV RNA 24 weeks after the end of treatment using a sensitive qualitative assay (TMA LOD <10 IU/mL). None of the subjects in the no-treatment arm of study IRHC-001 achieved an SVR.
Combined SVR results from IRHC-001 and IRHC-002 according to baseline characteristics are shown in Table 8. Based on these results, INFERGEN 15 mcg is the recommended starting dose.
|
INFERGEN 9 mcg/ribavirin |
INFERGEN 15 mcg/ribavirin |
|
|
Overall SVR |
5% (13/245) |
9% (21/242) |
|
Genotype 1 |
4% (10/231) |
6% (15/233) |
|
- F0-3 |
5% (9/181) |
7% (12/167) |
|
- F4 |
2% (1/50) |
5% (3/66) |
|
Other Genotypes |
21% (3/14) |
67% (6/9) |
|
- F0-3 |
27% (3/11) |
75% (6/8) |
|
- F4 |
0% (0/3) |
0% (0/1) |
|
HCV RNA <850,000 IU/mL |
13% (10/77) |
14% (11/78) |
|
HCV RNA ≥850,000 IU/mL |
2% (3/168) |
6% (10/163) |
|
Caucasian |
6% (10/158) |
10% (16/158) |
|
African American |
4% (2/52) |
5% (2/42) |
|
Other race |
3% (1/35) |
7% (3/42) |
16. How is Infergen supplied
Use only one vial per dose; do not re-enter the vial. Discard unused portions. Do not save unused drug for later administration.
Single-use, preservative-free vials containing 9 mcg (0.3 mL) of interferon alfacon-1 are available in dispensing packs of 6 vials (NDC 66435-202-09).
Single-use, preservative-free vials containing 15 mcg (0.5 mL) of interferon alfacon-1 are available in dispensing packs of 6 vials (NDC 66435-201-15).
INFERGEN should be stored in the refrigerator at 2 °C to 8 °C (36 °F to 46 °F). Do not freeze. Avoid vigorous shaking and exposure to direct sunlight
17. Patient Counseling Information
17.1 Information for Patients
Patients should be instructed on appropriate use by a health care professional. Patients receiving INFERGEN alone or in combination treatment with INFERGEN/ribavirin must be instructed as to the proper dosage and administration, and informed of the benefits and risks associated with treatment [see Medication Guide and Ribavirin Labeling]. Information included in the Medication Guide should be reviewed fully with the patient; it is not a disclosure of all or possible adverse reactions.
Patients must be informed that ribavirin may cause birth defects and/or death of the unborn child. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients during combination treatment with INFERGEN/ribavirin therapy and for 6 months post-therapy. Combination treatment with INFERGEN/ribavirin should not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. It is recommended that patients undergo monthly pregnancy tests during therapy and for 6 months post-therapy [see CONTRAINDICATIONS (4) and Ribavirin Labeling].
Patients should be informed that there are no data regarding whether INFERGEN therapy will prevent transmission of HCV infection to others. Also, it is not known if treatment with INFERGEN will cure hepatitis C or prevent cirrhosis, liver failure, or liver cancer that may be the result of infection with the hepatitis C virus.
The most common adverse reactions occurring with INFERGEN and combination treatment with INFERGEN/ribavirin are flu-like symptoms including fatigue, fever, nausea, headache, arthralgia, myalgia, rigors, and increased sweating. Non-narcotic analgesics and bedtime administration of INFERGEN may be used to prevent or lessen some of these symptoms. Other common adverse reactions are neutropenia, insomnia, leukopenia, and depression.
While fever may be related to the flu-like symptoms reported in patients treated with INFERGEN, when fever occurs, other possible causes of persistent fever should be ruled out.
Patients must be thoroughly instructed in the importance of proper disposal procedures and cautioned against the reuse of needles, syringes, or re-entry of the vial. A puncture-resistant container for the disposal of used syringes and needles should be used by the patient and should be disposed of according to the directions provided by the health care provider [see Medication Guide for instructions].
Patients should be advised that laboratory evaluations are required before starting therapy and periodically thereafter [see WARNINGS AND PRECAUTIONS: Laboratory Tests (5.16)]. It is advised that patients be well hydrated, especially during the initial stages of treatment.
Manufactured by:
Boehringer Ingelheim Pharma GmbH & Co.
Biberach, Germany
Manufactured for:
Kadmon Pharmaceuticals, LLC
Warrendale, PA 15086, USA
(877) 377-7862
This product and its use are covered by the following US Patent Nos.: 5,372,808; 5,541,293; 5,980,884.
C129.00008 v2.0
Medication Guide
INFERGEN®(Iń-fer-jen)
(interferon alfacon-1) Injection for subcutaneous use
Read this Medication Guide carefully before you start taking INFERGEN and each time you get a refill. There may be new information. This information does not take place of talking with your healthcare provider about your medical condition or treatment.
If you are taking INFERGEN with ribavirin, also read the Medication Guide for ribavirin capsules or tablets.
What is the most important information I should know about INFERGEN?
INFERGEN can cause serious side effects. Some of these side effects may cause death. Tell your healthcare provider right away if you have any of the symptoms listed below while taking INFERGEN.
1. Mental health problems and suicide: Some patients taking INFERGEN may develop mood or behavior problems, including:
- •
- irritability (getting upset easily)
- •
- depression (feeling hopeless or feeling bad about yourself)
- •
- nervousness
- •
- anxiety
- •
- aggressive behavior
- •
- former drug addicts may fall back into drug addiction or overdose
- •
- thoughts of hurting yourself or others, or suicide
2. New or worsening autoimmune problems. Some people taking INFERGEN develop autoimmune problems (a condition where the body’s immune cells attack other cells or organs in the body), including rheumatoid arthritis, systemic lupus erythematosus and psoriasis. In some people who already have an autoimmune problem, it may get worse during your treatment with INFERGEN.
3. Heart problems: Some people who take INFERGEN may get heart problems, including:
- •
- low blood pressure
- •
- fast heart beat or abnormal heart beat
- •
- chest pains
- •
- heart attack or heart muscle problem (cardiomyopathy)
4. Stroke or symptoms of a stroke. Symptoms may include weakness, loss of coordination, and numbness. Stroke or symptoms of a stroke may happen in people who have some risk factors or no known risk factors for a stroke.
5. Infections. Some people who take INFERGEN may get an infection. Symptoms may include:
|
• fever |
• urinating often |
|
• chills |
• bloody diarrhea |
|
• pain and/or burning with urination |
• coughing up mucous |
Before taking INFERGEN, tell your healthcare provider right away if you:
- •
- are being treated for a mental illness or had treatment in the past for any mental illness, including depression and suicidal behavior
- •
- have or ever had any problems with your heart, including heart attack or high blood pressure
- •
- have any kind of autoimmune disease (where the body’s immune system attacks the body’s own cells), such as psoriasis, systemic lupus erythematosus, rheumatoid arthritis
- •
- have or ever had bleeding or a blood clot
- •
- have or ever had low blood cell counts
- •
- have ever been addicted to drugs or alcohol
Call your healthcare provider right away if you get any of these problems while taking INFERGEN:
- •
- new or worse mental health problems, such as thoughts of hurting yourself or others, or suicide
- •
- trouble breathing or severe chest pain
- •
- any new weakness, loss of coordination, or numbness
- •
- fever, chills, burning and/or pain with urination, urinating often, bloody diarrhea
While taking INFERGEN, you should see a healthcare provider regularly for check-ups and blood tests to make sure that your treatment is working, and to check for side effects.
What is INFERGEN?
INFERGEN (interferon alfacon-1) is a prescription medicine used to treat adults with lasting chronic (lasting a long time) hepatitis C virus (HCV) infection and certain types of liver problems.
It is not known if INFERGEN is safe and will work if taken for more than 1 year.
It is not known if INFERGEN is safe and will work in people younger than 18 years old.
Who should not take INFERGEN?
Do not take INFERGEN if you:
- •
- have certain types of other liver problems
- •
- have certain types of hepatitis (autoimmune hepatitis)
- •
- have had a serious allergic reaction to another alpha-interferon medicine or to any of the ingredients in INFERGEN. See the end of this Medication Guide for a complete list of the ingredients. Symptoms of a serious allergic reaction to alpha-interferon may include: itching, swelling of your face, tongue, throat, trouble breathing, feeling dizzy or faint, and chest pain.
Talk to your healthcare provider before taking INFERGEN if you have any of these conditions.
What should I tell my healthcare provider before taking INFERGEN?
Before you take INFERGEN, See “What is the most important information I should know about INFERGEN?” and tell your healthcare provider if you have:
- •
- Liver problems (other than hepatitis C infection)
- •
- Have or had lung problems such as chronic obstructive pulmonary disease (COPD)
- •
- Thyroid problems
- •
- Diabetes
- •
- Colitis (inflammation of your intestine)
- •
- Cancer
- •
- Hepatitis B infection
- •
- HIV infection
- •
- Kidney problems
- •
- Have high blood triglyceride levels (fat in your blood)
- •
- Organ transplant and are taking medicine that keeps your body from rejecting your transplant (suppresses your immune system)
- •
- Any other medical conditions
- •
- You are pregnant or plan to become pregnant. It is not known if INFERGEN will harm your unborn baby. You should use effective birth control during treatment with INFERGEN. Talk to your healthcare provider about birth control choices for you during treatment with INFERGEN. Tell your healthcare provider if you become pregnant during treatment with INFERGEN.
- •
- Are breast feeding or plan to breast-feed. It is not known if INFERGEN passes into your breast milk. You and your healthcare provider should decide if you will use INFERGEN or breast-feed.
Tell your healthcare provider about all the medicines you take including prescription or non-prescription medicines, vitamin and mineral supplements and herbal medicines. INFERGEN and certain other medicines may affect each other and cause side effects.
Especially tell your healthcare provider if you take the anti-hepatitis B medicine telbivudine (Tyzeka). Some people who take this medicine with INFERGEN develop nerve problems (peripheral neuropathy), such as continuing numbness, tingling, or burning feeling in the arms or legs, or problems walking.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
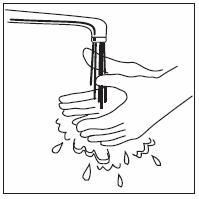
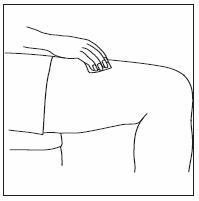
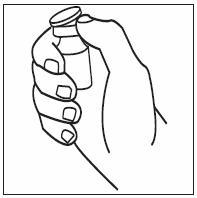
How should I take INFERGEN?
- •
- Take INFERGEN exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much INFERGEN to take and when to take it. Do not take more than your prescribed dose.
- •
- Your healthcare provider will decide whether you will take INFERGEN by itself three times a week, or everyday with ribavirin.
- •
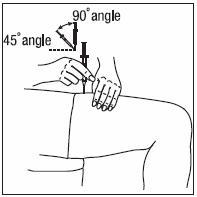
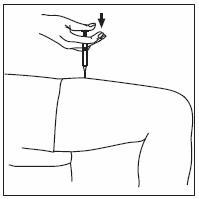
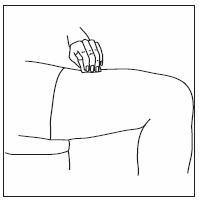
- INFERGEN is given as an injection under your skin (subcutaneous injection). Your healthcare provider should show you how to prepare and measure your dose of INFERGEN, and how to inject your INFERGEN yourself before you use INFERGEN for the first time.
- •
- You should not inject INFERGEN until your healthcare provider has shown you how to use INFERGEN the right way.
- •
- INFERGEN comes in single ready-to-use vials. There is 1 dose of medicine in each vial. Do not change your dose unless your healthcare provider tells you to change it. It is important that you take INFERGEN exactly as your healthcare provider tells you. Too little INFERGEN may not be effective in treating your HCV infection and too much INFERGEN may cause side effects.
- •
- Inject your dose of INFERGEN as prescribed, at the same time of day.
- •
- If you miss a dose of INFERGEN, give yourself an injection as soon as you remember and then call your healthcare provider. Do not take your next scheduled dose until you have been told what you should do by your healthcare provider.
- •
- If you take more than your prescribed amount of INFERGEN, call your healthcare provider right away. Your healthcare provider may want to examine you.
What are the possible side effects of INFERGEN?
Your healthcare provider should do regular blood tests before you start INFERGEN, and during treatment to see how the treatment is working and to check for side effects.
INFERGEN may cause serious side effects including:
- •
- See “What is the most important information I should know about INFERGEN?”
- •
-
Lung problems including:
- •
- Trouble breathing
- •
- Pneumonia
- •
- Inflammation of lung tissue
- •
- New or worse high blood pressure of the lungs (pulmonary hypertension). This can be severe and may lead to death.
- •
-
Severe liver problems, or worsening of liver problems, including liver failure and death. Symptoms may include:
- •
- Nausea
- •
- Loss of appetite
- •
- Tiredness
- •
- Diarrhea
- •
- Yellowing of your skin or the white part of your eyes
- •
- Bleeding more easily than normal
- •
- Swelling of your stomach area (abdomen)
- •
- Confusion
- •
- Sleepiness
- •
- You cannot be awakened (coma)
- •
-
Swelling of your pancreas (pancreatitis), intestines (colitis), or kidneys.
Symptoms may include:- •
- Severe stomach area (abdomen) pain
- •
- Severe back pain
- •
- Nausea and vomiting
- •
- Bloody diarrhea or bloody bowel movements
- •
- Fever
- •
- Blood problems. INFERGEN can affect your bone marrow and cause low white blood cell and platelet counts. In some people, these blood counts may fall to dangerously low levels. If your blood counts become very low, you can get infections, and problems with bleeding and bruising.
- •
- Serious allergic reactions and skin reactions. Symptoms may include:
|
• Itching • Swelling of the face, eyes, lips, tongue, or throat • Trouble breathing • Anxiousness |
• Chest pain • Feeling faint • Skin rash, hives, sores in your mouth, or your skin blisters and peels |
- •
- Serious eye problem. INFERGEN may cause eye problems that may lead to vision loss or blindness. You should have an eye exam before you start taking INFERGEN. If you have eye problems or have had them in the past, you may need eye exams right away if you are taking INFERGEN. Tell your healthcare provider or eye doctor right away if you have any vision changes while taking INFERGEN.
- •
- Nerve problems: People who take INFERGEN or other interferon alpha products with telbivudine (Tyzeka) can have nerve problems such as continuing numbness, tingling, or burning sensation in the arms or legs (peripheral neuropathy). Call your healthcare provider if you have any of these symptoms.
- •
-
Thyroid problems. Some people develop changes in their thyroid function. Symptoms of thyroid changes include:
• Problems concentrating
• Feeling cold or hot all of the time
• Weight changes
• Skin changes - •
-
Blood sugar problems. Some people may develop high blood sugar or diabetes. If you have high blood sugar or diabetes that is not controlled before starting INFERGEN, talk to your healthcare provider before you take INFERGEN. If you develop high blood sugar or diabetes while taking INFERGEN, your healthcare provider may tell you to stop INFERGEN and prescribe a different medicine for you. Symptoms of high blood sugar or diabetes may include:
• Increased thirst
• Tiredness
• Urinating more often than normal
• Increased appetite
• Weight loss
• Your breath smells like fruit
Tell your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects of INFERGEN include:
- •
- Flu-like symptoms. Symptoms may include: headache, muscle aches, tiredness, chills and fever. Some of these symptoms may be decreased by injecting your INFERGEN dose at bedtime. Talk to your healthcare provider about other over-the-counter medicines that you can take to help prevent or decrease some of these symptoms.
- •
- Tiredness. Many people become very tired during treatment with INFERGEN.
- •
- Stomach problems. Nausea, loss of appetite, diarrhea and weight loss may happen with INFERGEN.
- •
- Hair thinning
Tell your healthcare provider if you have any side effects that bother you or does not go away.
These are not all of the side effects of INFERGEN. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store INFERGEN?
- •
- Store INFERGEN in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC).
- •
- Do not freeze INFERGEN. Do not use a vial of INFERGEN that has been frozen.
- •
- Keep INFERGEN away from direct sunlight.
- •
- Do not use a vial of INFERGEN past the expiration date stamped on the label.
- •
- Do not shake INFERGEN. If INFERGEN is shaken too hard, it will not work properly.
Keep INFERGEN and all medicines out of the reach of children.
General information about INFERGEN
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use INFERGEN for a condition for which it was not prescribed. Do not give INFERGEN to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about INFERGEN. If you would like more information, ask your healthcare provider. You can ask your healthcare provider or pharmacist for information about INFERGEN that was written for healthcare professionals.
For more information, go to www.infergen.com.
What are the ingredients in INFERGEN?
Active Ingredients: interferon alfacon-1
Inactive ingredients: sodium chloride, and sodium phosphate in Water for Injection.
Manufactured by:
Boehringer Ingelheim Pharma GmbH & Co.
Biberach, Germany
Manufactured for: Kadmon Pharmaceuticals, LLC
Warrendale, PA 15086, USA
(877) 377-7862
C002.00021
This Medication Guide has been approved by the U.S. Food and Drug Administration.
INFERGEN Instructions for Use
Instructions for Use
INFERGEN® (Iń-fer-jen)
(interferon alfacon-1)
Injection
Be sure that you read, understand and follow these instructions before injecting INFERGEN. Before you use it for the first time, your healthcare provider should show you how to prepare, measure and inject INFERGEN properly. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting INFERGEN. For each injection, you will need an INFERGEN vial package that contains:
- •
- A vial of INFERGEN
- •
- One sterile disposable syringe and needle
- •
- Several alcohol swabs and
- •
- A puncture-proof container to dispose of the needle and syringe when you are done
Important:
- •
- Never re-use your disposable syringes and needles.
- •
- Each vial is for a single-use. Throw away the vial after you use it 1 time.
- •
- Make sure you have the right syringe to use with INFERGEN. It is important to use a syringe that is marked in tenths of milliliters (mLs), for example, 0.1 mL. Your healthcare provider may refer to a mL as a cc (1 mL= 1cc).
How should I prepare a dose of INFERGEN?
1. Find a clean, well-lit, flat working surface. Remove a vial of INFERGEN from the refrigerator. Right before your injection, INFERGEN may be allowed to reach room temperature.
2. Check the date on the vial of INFERGEN and make sure that the date has not passed. Do not use the vial of INFERGEN if the expiration date has passed.
3. Look at the liquid inside the vial of INFERGEN. The liquid in the vial should be clear and colorless.
Do not use the INFERGEN if the liquid:
- •
- is cloudy
- •
- is not clear and colorless
- •
- has particles in it
4. Wash your hands well with soap and water.
Select and prepare the injection site on your body
5. Pick a site for your injection. You should change the site for injection each time you inject to avoid soreness at any one site.
6. Clean the injection site with an alcohol swab. Use circular motions from the inside to the outside. Keep the used alcohol swab nearby.
Preparing a dose of INFERGEN
7. Remove the colored cap from the vial, exposing the rubber stopper.
8. Clean the rubber stopper with a new alcohol swab, and then cover the stopper with the swab.
9. Remove the syringe and needle from their packages. If either package looks like it has been opened or damaged, do not use the syringe or needle; dispose of it in the puncture-proof disposal container.
10. Remove the needle cover and pull the plunger back and draw air into the syringe. The amount of air you draw into the syringe should be the same amount as the dose of medicine your healthcare provider has prescribed.
11. Remove the alcohol wipe from the top of the vial and insert the needle straight through the center of the rubber stopper.
12. Push the plunger of the syringe down to inject the air into the air space above the liquid in the vial. The air injected into the vial will allow Infergen to be easily withdrawn from the vial into the syringe.
13. Keep the needle in the vial, turn the vial upside down and make sure that the tip of the needle is in the liquid.
14. Slowly pull the plunger back and let the medicine enter the syringe, filling it to the line that equals the dose your healthcare provider prescribed.
15. Keep the needle in the vial. Check for air bubbles in the syringe. Small air bubbles are harmless but can reduce the dose of INFERGEN that you receive.
- •
- To remove the air bubbles, gently tap the syringe with your fingers until the bubbles rise to the needle-end of the syringe barrel.
- •
- Then push the plunger in to force the air out of the syringe.
- •
- Make sure the tip of the needle is in the liquid and slowly pull back on the plunger until the liquid in the syringe reaches the mark that correctly matches the amount of your dose.
16. Take the needle out of the vial and hold the syringe needle facing up in the hand that you will use to inject yourself. Do not lay the syringe down or allow the needle to touch anything.
Injecting a dose of INFERGEN
17. Use the other hand to pinch a fold of skin at the site you cleaned for an injection.
18. Hold the syringe the way you would hold a pencil and insert the needle into your skin either straight up and down (90 degree angle) or at a slight angle (45 degree angle) to the skin.
19. After the needle is inserted, let go of the skin.
- •
- Pull the plunger back slightly.
- •
-
If blood comes into the syringe, do not inject INFERGEN, because the needle has entered a blood vessel.
- •
- Withdraw the syringe and needle. Discard the syringe and needle in the puncture-proof container. See step “How should I dispose of used syringes and needles?”
- •
- Then repeat steps 1 through 19 to prepare a new dose of INFERGEN with a new syringe and needle, and inject the dose at a new site.
- •
- If no blood comes into the syringe, slowly push down on the plunger all the way, until all the medicine is injected and the syringe is empty.
20. Pull the needle out of the skin at the same angle you put it in and:
- •
- Place a cotton ball or gauze over the injection site and press for several seconds. Do not massage the injection site.
- •
- If there is bleeding, cover the injection site with a bandage.
21. Place the needle and syringe in the puncture-proof disposal container right away. Never reuse the syringe or needle. Do not recap the needle. See “How should I dispose of the used syringes, needles, and vials?”
How should I dispose of used syringes, needles, and vials?
- •
- Throw away used syringes, needles, and vials in a closable, puncture-proof container, sharps container, or a hard container such as a coffee can.
- •
- Check with your healthcare provider or pharmacist about the right way to throw away used needles and syringes. There may be state and local laws about how you should dispose of used needles and syringes.
Always keep the container out of the reach of children.
Do not recycle containers or throw full containers into the household trash.
How should I store INFERGEN?
- •
- Store INFERGEN in the refrigerator at 36 °F to 46 °F (2 °C to 8 °C).
- •
- Do not freeze INFERGEN. Do not use a vial of INFERGEN that has been frozen.
- •
- Keep INFERGEN away from direct sunlight.
- •
- Do not use a vial of INFERGEN past expiration date stamped on the label.
- •
- To transport Infergen‚ keep the vials cool and avoid extreme temperature changes.
- •
- Do not shake INFERGEN. If INFERGEN is shaken too hard, it will not work properly.
Keep INFERGEN and all medicines out of the reach of children.
Manufactured by:
Boehringer Ingelheim Pharma GmbH & Co.
Biberach, Germany
Manufactured for:
Kadmon Pharmaceuticals, LLC
Warrendale, PA 15086, USA
(877) 377-7862
This product and its use are covered by the following US Patent Nos.: 5,372,808; 5,541,293; 5,980,884.
C002.00021
PRINCIPAL DISPLAY PANEL - Vial - 9 mcg
NDC 66435-202-95 Refrigerate at 2 ° to 8 °C
Rx only
Infergen®
(Interferon alfacon-1)
0.3 mL Single-Use Vial 9 mcg/0.3 mL
Kadmon
Kadmon Pharmaceuticals, LLC.
Warrendale, PA 15086
U.S. License No. 1802
C002.00024
72601-02
Lot
Exp.
PRINCIPAL DISPLAY PANEL - Carton - 9 mcg
Top Panel
NDC-66435-202-09 6-0.3 mL Single-Use Vials
Rx only
Infergen®
(Interferon alfacon-1)
A recombinant consensus
alpha interferon
For Subcutaneous Use Only
Sterile Solution - No Preservative
Ready to Use
Refrigerate at 2 ° to 8 °C (36 ° to 46 °F).
Do Not Freeze or Shake.
Kadmon
Pharmaceuticals
Medication Guide for
patients enclosed
9 mcg/0.3 mL
Front Panel
Infergen®9 mcg/0.3 mL
(Interferon alfacon-1)
A recombinant consensus alpha interferon
For Subcutaneous Use Only
Sterile Solution - No Preservative
Kadmon Pharmaceuticals, LLC.
Warrendale, PA 15086, USA
U.S. License No. 1802
Kadmon
Pharmaceuticals
Side Panels
Infergen®
(Interferon alfacon-1)
9 mcg/0.3 mL
6 - 0.3 mL
Single-Use Vials
No U.S. standard of potency
Back Panel
Infergen®9 mcg/0.3 mL
(Interferon alfacon-1)
Each 0.3 mL vial contains 9 mcg Interferon
alfacon-1 in a sterile, preservative-free solution
(pH 7.0 ± 0.2) containing sodium chloride (1.77 mg) and
sodium phosphate (1.14 mg) in Water for Injection, USP.
Dosage: See package insert for full information.
Refrigerate at 2 ° to 8 °C (36 ° to 46 °F).
Do Not Freeze or Shake.
U.S. Patent Nos. 5,372,808; 5,541,293; 5,980,884
ATTENTION PHARMACIST:
Each patient is required to receive the enclosed Medication Guide
Bottom Panel
C002.00022
72605-02
PRINCIPAL DISPLAY PANEL - Vial - 15 mcg
NDC 66435-201-96 Refrigerate at 2 ° to 8 °C
Rx only
Infergen®
(Interferon alfacon-1)
0.5 mL Single-Use Vial 15 mcg/0.5 mL
Kadmon
Kadmon Pharmaceuticals, LLC.
Warrendale, PA 15086
U.S. License No. 1802
C002.00025
72602-02
Lot
Exp.
PRINCIPAL DISPLAY PANEL - Carton - 15 mcg
Top Panel
NDC-66435-201-15 6-0.5 mL Single-Use Vials
Rx only
Infergen®
(Interferon alfacon-1)
A recombinant consensus
alpha interferon
For Subcutaneous Use Only
Sterile Solution - No Preservative
Ready to Use
Refrigerate at 2 ° to 8 °C (36 ° to 46 °F).
Do Not Freeze or Shake.
Kadmon
Pharmaceuticals
Medication Guide for
patients enclosed
15 mcg/0.5 mL
Front Panel
Infergen®15 mcg/0.5 mL
(Interferon alfacon-1)
A recombinant consensus alpha interferon
For Subcutaneous Use Only
Sterile Solution - No Preservative
Kadmon Pharmaceuticals, LLC.
Warrendale, PA 15086, USA
U.S. License No. 1802
Kadmon
Pharmaceuticals
Side Panels
Infergen®
(Interferon alfacon-1)
15 mcg/0.5 mL
6 - 0.5 mL
Single-Use Vials
No U.S. standard of potency
Back Panel
Infergen®15 mcg/0.5 mL
(Interferon alfacon-1)
Each 0.5 mL vial contains 15 mcg Interferon
alfacon-1 in a sterile, preservative-free solution
(pH 7.0 ± 0.2) containing sodium chloride (2.95 mg) and
sodium phosphate (1.90 mg) in Water for Injection, USP.
Dosage: See package insert for full information.
Refrigerate at 2 ° to 8 °C (36 ° to 46 °F).
Do Not Freeze or Shake.
U.S. Patent Nos. 5,372,808; 5,541,293; 5,980,884
ATTENTION PHARMACIST:
Each patient is required to receive the enclosed Medication Guide
Bottom Panel
C002.00023
72606-02
| INFERGEN
interferon alfacon-1 injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| INFERGEN
interferon alfacon-1 injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kadmon Pharmaceuticals, LLC (843913216) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 340700520 | MANUFACTURE(66435-202, 66435-201) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim RCV GmbH & Co KG | 300010883 | MANUFACTURE(66435-202, 66435-201) | |
More about Infergen (interferon alfacon-1)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: interferons
- Breastfeeding