Xolair Dosage
Generic name: OMALIZUMAB 150mg in 1.2mL
Dosage form: injection, solution
Drug class: Selective immunosuppressants
Medically reviewed by Drugs.com. Last updated on Feb 22, 2024.
Overview of Dosage Determination
Asthma, and Chronic Rhinosinusitis with Nasal Polyps, and IgE-Mediated Food Allergy
- Determine dosage of XOLAIR by serum total IgE level (IU/mL) measured before the start of treatment, and by body weight (kg).
- For patients with asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and IgE-mediated food allergy, dosage determination should be based on the primary diagnosis for which XOLAIR is being prescribed.
- Adjust doses for significant changes in body weight during treatment.
- Refer to Tables 1 and 2 for the recommended dosage for treatment of asthma, Table 3 for treatment of CRSwNP, and Table 4 for treatment of IgE-mediated food allergy.
- Total IgE levels are elevated during treatment and remain elevated for up to one year after the discontinuation of treatment. Therefore, re-testing of IgE levels during XOLAIR treatment cannot be used as a guide for dose determination.
- Interruptions lasting less than one year: Dose based on serum IgE levels obtained at the initial dose determination.
- Interruptions lasting one year or more: Re-test total serum IgE levels for dose determination (Table 1 or 2 for treatment of asthma, based on the patient's age, Table 3 for treatment of CRSwNP, and Table 4 for treatment of IgE-mediated food allergy).
Chronic Spontaneous Urticaria
Dosage of XOLAIR in patients with chronic spontaneous urticaria (CSU) is not dependent on serum IgE (free or total) level or body weight [see Dosage and Administration (2.5)].
Recommended Dosage for Asthma
The recommended dosage for asthma is XOLAIR 75 mg to 375 mg by subcutaneous injection every 2 or 4 weeks based on serum total IgE level (IU/mL) measured before the start of treatment and by body weight (kg) [see Dosage and Administration (2.1)].
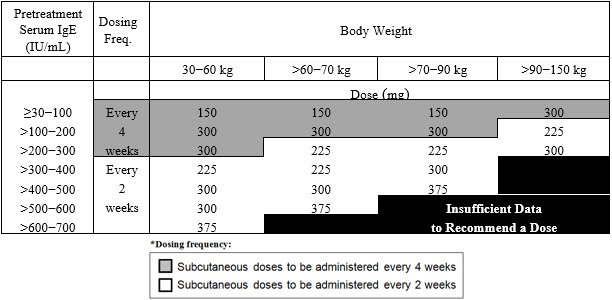
- Adult and adolescent patients 12 years of age and older: Initiate dosing according to Table 1.
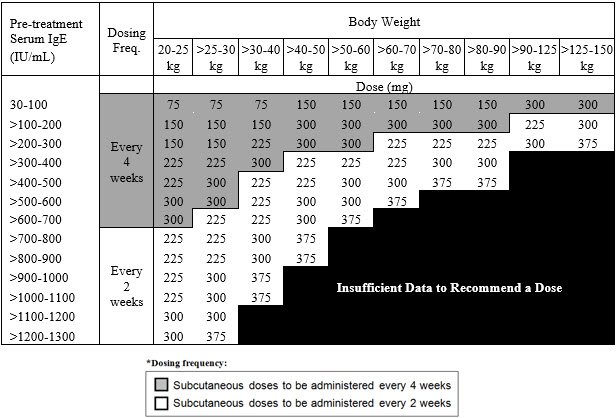
- Pediatric patients 6 to <12 years of age: Initiate dosing according to Table 2.
 |
 |
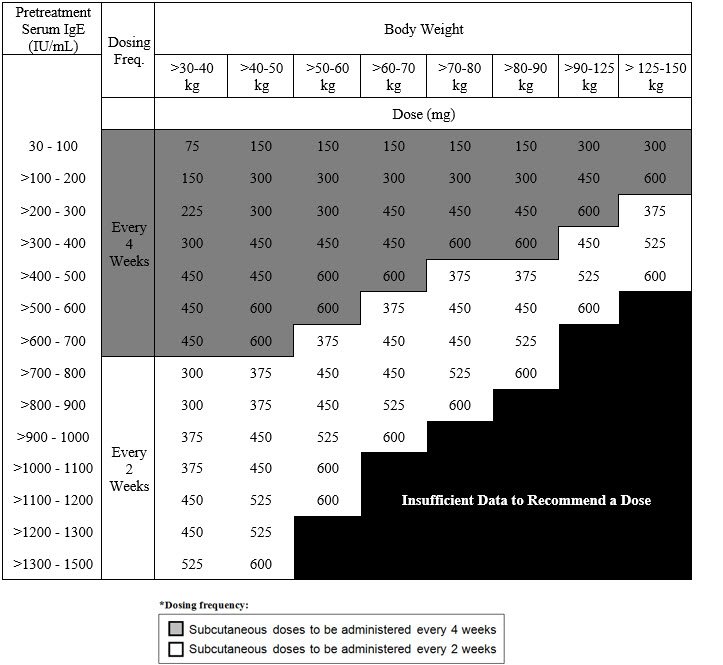
Recommended Dosage for Chronic Rhinosinusitis with Nasal Polyps
The recommended dosage for chronic rhinosinusitis with nasal polyps (CRSwNP) is XOLAIR 75 mg to 600 mg by subcutaneous injection every 2 or 4 weeks based on serum total IgE level (IU/mL) measure before the start of treatment and by body weight (kg) [see Dosage and Administration (2.1)]. Refer to Table 3 for recommended dosage based on serum total IgE level and body weight for patients with CRSwNP.
 |
Recommended Dosage for IgE-Mediated Food Allergy
The recommended dosage for IgE-mediated food allergy is XOLAIR 75 mg to 600 mg by subcutaneous injection every 2 or 4 weeks based on serum total IgE level (IU/mL), measured before the start of treatment, and by body weight [see Dosage and Administration (2.1)]. Refer to Table 4 for recommended dosage based on serum IgE level and body weight for patients with IgE-mediated food allergy.
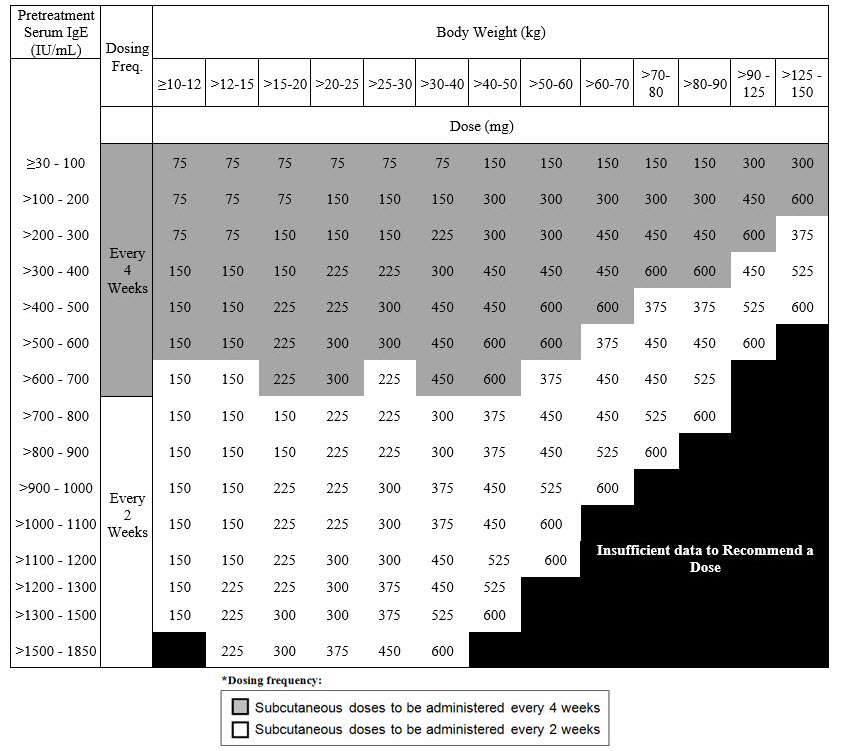 |
Recommended Dosage for Chronic Spontaneous Urticaria
The recommended dosage for chronic spontaneous urticaria (CSU) is XOLAIR 150 mg or 300 mg by subcutaneous injection every 4 weeks.
- The 300 mg dose may be administered as one subcutaneous injection of 300 mg/2 mL or as two subcutaneous injections of 150 mg/mL.
- Dosing of XOLAIR in CSU patients is not dependent on serum IgE (free or total) level or body weight.
Administration Overview
- Administer XOLAIR by subcutaneous injection.
- XOLAIR is intended for use under the guidance of a healthcare provider.
- Initiate therapy in a healthcare setting and once therapy has been safely established, the healthcare provider may determine whether self-administration of XOLAIR prefilled syringe or autoinjector by the patient or caregiver is appropriate, based on careful assessment of risk for anaphylaxis and mitigation strategies.
Selection of Patients for Self-Administration of XOLAIR Prefilled Syringe or Autoinjector
Healthcare providers should consider known risk factors for anaphylaxis to XOLAIR [see Warnings and Precautions (5.1)] and mitigation strategies when selecting patients for self-administration. Patient-specific factors including the following criteria should be considered:
- 1a)
- Asthma, CRSwNP and CSU: Patient should have no prior history of anaphylaxis to XOLAIR or other agents, such as foods, drugs, biologics, etc.
- 1b)
- IgE-Mediated Food Allergy: Patient should have no prior history of anaphylaxis to XOLAIR or other agents (except foods), such as drugs, biologics, etc.
- 2)
- Patient should receive at least 3 doses of XOLAIR under the guidance of a healthcare provider with no hypersensitivity reactions
- 3)
- Patient or caregiver is able to recognize symptoms of anaphylaxis
- 4)
- Patient or caregiver is able to treat anaphylaxis appropriately
- 5)
- Patient or caregiver is able to perform subcutaneous injections with XOLAIR prefilled syringe or autoinjector with proper technique according to the prescribed dosing regimen and Instructions for Use
XOLAIR Prefilled Syringe and Autoinjector
XOLAIR injection doses are available as a prefilled syringe or as an autoinjector. Instruct patients or caregivers to follow the directions provided in the "Instructions for Use" for preparation and administration of XOLAIR prefilled syringe or autoinjector [see Instructions for Use].
XOLAIR Prefilled Syringe
- Adolescents 12 years of age and older: XOLAIR prefilled syringe may be self-administered under adult supervision.
- Pediatric Patients 1 to 11 years of age: XOLAIR prefilled syringe should be administered by a caregiver.
XOLAIR Autoinjector
- Adolescents 12 years of age and older: XOLAIR autoinjector may be self-administered under adult supervision. The XOLAIR autoinjectors (all doses) are intended for use only in adults and adolescents aged 12 years and older.
- Pediatric Patients 1 to 11 years of age: The XOLAIR autoinjectors (all doses) are not intended for use in pediatric patients under 12 years of age.
Administration Instructions for Prefilled Syringe and Autoinjector
- Persons with latex allergies should not handle XOLAIR prefilled syringe because the needle cap of the XOLAIR 75 mg/0.5 mL and 150 mg/mL prefilled syringes contains a derivative of natural rubber latex which may cause allergic reactions in latex sensitive individuals [see How Supplied/Storage and Handling (16)].
- Visually inspect the contents of the prefilled syringe or autoinjector for particulate matter and discoloration prior to administration. XOLAIR prefilled syringe or autoinjector solution should be clear and colorless to pale brownish yellow. Do not use the prefilled syringe or autoinjector if the medicine is cloudy, discolored, or contains particles.
- Determine the number of prefilled syringes or autoinjectors needed for patient's dosage (see Table 5). For pediatric patients 1 to 11 years of age, consideration should be given to the number of prefilled syringe injections needed and volume to be injected relative to the patient's bodyweight.
- For patients requiring more than 1 injection to complete a full dose, administer each injection at least 1 inch apart from other injection sites.
- Administer subcutaneous injection into the thigh or abdomen, avoiding the 2-inch (5 cm) area directly around the navel. The outer area of the upper arms may be used only if the injection is being given by a caregiver or healthcare provider [see Instructions for Use]. The injection may take up to 15 seconds to administer.
| XOLAIR Dose‡ | 75 mg | 150 mg | 300mg‡ | Total Volume Injected |
|---|---|---|---|---|
|
||||
| 75 mg | 1 | 0 | 0 | 0.5 mL |
| 150 mg | 0 | 1 | 0 | 1 mL |
| 225 mg | 1 | 1 | 0 | 1.5 mL |
| 300 mg | 0 | 0 | 1 | 2 mL |
| 375 mg | 1 | 0 | 1 | 2.5 mL |
| 450 mg | 0 | 1 | 1 | 3 mL |
| 525 mg | 1 | 1 | 1 | 3.5 mL |
| 600 mg | 0 | 0 | 2 | 4 mL |
Preparation for Use and Injection of XOLAIR Lyophilized Powder
XOLAIR lyophilized powder should only be prepared and injected by a healthcare provider. The supplied XOLAIR lyophilized powder must be reconstituted with Sterile Water for Injection (SWFI) USP, using the following instructions:
- 1)
- Before reconstitution, determine the number of vials that will need to be reconstituted (each vial delivers 150 mg of XOLAIR in 1.2 mL) (see Table 6).
| XOLAIR Dose* | Number of Vials | Number of Injections | Total Volume Injected |
|---|---|---|---|
|
|||
| 75 mg | 1 | 1 | 0.6 mL |
| 150 mg | 1 | 1 | 1.2 mL |
| 225 mg | 2 | 2 | 1.8 mL |
| 300 mg | 2 | 2 | 2.4 mL |
| 375 mg | 3 | 3 | 3.0 mL |
| 450 mg | 3 | 3 | 3.6 mL |
| 525 mg | 4 | 4 | 4.2 mL |
| 600 mg | 4 | 4 | 4.8 mL |
- 2)
- Draw 1.4 mL of SWFI, USP, into a 3 mL syringe equipped with a 1-inch, 18-gauge needle.
- 3)
- Place the vial upright on a flat surface and using standard aseptic technique, insert the needle and inject the SWFI, USP, directly onto the product.
- 4)
- Keeping the vial upright, gently swirl the upright vial for approximately 1 minute to evenly wet the powder. Do not shake.
- 5)
- Gently swirl the vial for 5 to 10 seconds approximately every 5 minutes in order to dissolve any remaining solids. The lyophilized product takes 15 to 20 minutes to dissolve. If it takes longer than 20 minutes to dissolve completely, gently swirl the vial for 5 to 10 seconds approximately every 5 minutes until there are no visible gel-like particles in the solution. Do not use if the contents of the vial do not dissolve completely by 40 minutes.
- 6)
- After reconstitution, XOLAIR solution is somewhat viscous and will appear clear or slightly opalescent. It is acceptable if there are a few small bubbles or foam around the edge of the vial; there should be no visible gel-like particles in the reconstituted solution. Do not use if foreign particles are present.
- 7)
- Invert the vial for 15 seconds in order to allow the solution to drain toward the stopper.
- 8)
- Use the XOLAIR solution within 8 hours following reconstitution when stored in the vial at 2ºC to 8ºC (36ºF to 46ºF), or within 4 hours of reconstitution when stored at room temperature. Reconstituted XOLAIR vials should be protected from sunlight.
- 9)
- Using a new 3 mL syringe equipped with a 1-inch, 18-gauge needle, insert the needle into the inverted vial. Position the needle tip at the very bottom of the solution in the vial stopper when drawing the solution into the syringe. The reconstituted product is somewhat viscous. Withdraw all of the product from the vial before expelling any air or excess solution from the syringe. Before removing the needle from the vial, pull the plunger all the way back to the end of the syringe barrel in order to remove all of the solution from the inverted vial.
- 10)
- Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection.
- 11)
- Expel air, large bubbles, and any excess solution in order to obtain a volume of 1.2 mL corresponding to a dose of 150 mg of XOLAIR. To obtain a volume of 0.6 mL corresponding to a dose of 75 mg of XOLAIR, expel air, large bubbles and discard 0.6 mL from the syringe. A thin layer of small bubbles may remain at the top of the solution in the syringe.
- 12)
- Administer XOLAIR by subcutaneous injection. The injection may take 5-10 seconds to administer because the solution is slightly viscous. Do not administer more than 150 mg (contents of one vial) per injection site. Divide doses of more than 150 mg between two or more injection sites. Choose a different injection site for each new injection at least 1 inch from the area used for other injections.
Frequently asked questions
- What are the most common skin conditions? (with photos)
- What are monoclonal antibodies?
- Is Xolair an immunosuppressant?
- How long before Xolair starts working?
- Does Xolair cause hair loss?
- Does Xolair cause cancer?
- How does Xolair work?
- Xolair and Covid-19 vaccine, what should I know?
- How long can Xolair stay out of the fridge?
More about Xolair (omalizumab)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (218)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- FDA approval history
- Drug class: selective immunosuppressants
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.