Mononine Dosage
Generic name: coagulation factor IX human 500[iU] in 5mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Feb 11, 2025.
MONONINE is intended for intravenous administration only. It should be reconstituted with the volume of Sterile Water for Injection, USP supplied with the lot, and administered within three hours of reconstitution. Do not refrigerate after reconstitution. After administration, any unused solution and the administration equipment should be discarded.
As a general rule, 1 IU of Factor IX activity per kg can be expected to increase the circulating level of Factor IX by 1% [IU/dL] of normal. The following formula provides a guide to dosage calculations:
| Number of Factor IX | = | Body Weight | × | desired Factor IX | × | 1.0 IU/kg |
| IU required (IU) | (in kg) | increase (% or IU/dL normal) | [per IU/dL] | |||
The amount of MONONINE to be infused, as well as the frequency of infusions, will vary with each patient and with the clinical situation.11,12
As a general rule, the level of Factor IX required for treatment of different conditions is as follows:
| Minor Spontaneous Hemorrhage, Prophylaxis | Major Trauma or Surgery | |
|---|---|---|
| Desired levels of Factor IX for Hemostasis | 15-25% [or IU/dL] |
25-50% [or IU/dL] |
| Initial loading dose to achieve desired level | up to 20-30 IU/kg | up to 75 IU/kg |
| Frequency of dosing | once; repeated in 24 hours if necessary | every 18-30 hours, depending on T1/2 and measured Factor IX levels |
| Duration of treatment | once; repeated if necessary | up to ten days, depending upon nature of insult |
Recovery of the loading dose varies from patient to patient. Doses administered should be titrated to the patient's response. MONONINE administered in doses of ≥75 IU/kg were well tolerated (see CLINICAL PHARMACOLOGY).
In the presence of an inhibitor to Factor IX, higher doses of MONONINE might be necessary to overcome the inhibitor (see PRECAUTIONS). No data on the treatment of patients with inhibitors to Factor IX with MONONINE are available.
For information on rate of administration, see Rate of Administration, below.
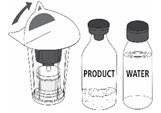
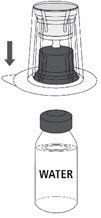
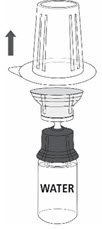
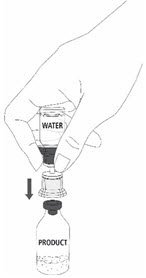
Reconstitution
Follow the steps provided below for the preparation and reconstitution of MONONINE:
- Check the expiration date on the vial label and carton. Do not use MONONINE after the expiration date.
- Check packaging for damage. Do not use MONONINE if the packaging is damaged or appears to be tampered with.
- Reconstitute MONONINE using aseptic technique to maintain product sterility.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
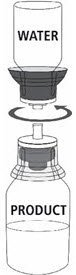 Fig. 5 |
|
 Fig. 6 |
| 12a. Draw air into an empty, sterile syringe. 12b. While the MONONINE vial is upright, connect the syringe to the transfer set Luer Lock fitting by screwing clockwise. Inject air into the MONONINE vial (Fig. 7). |
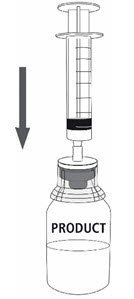 Fig. 7 |
|
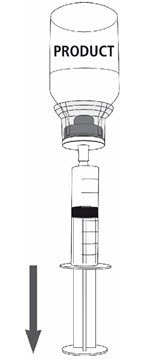 Fig. 8 |
|
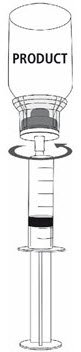 Fig. 9 |
Administration
Intravenous Injection
- Visually inspect the final solution for particles and discoloration prior to administration, and whenever solution and container permit. Do not use if particles or discoloration is observed.
- Attach the syringe containing the reconstituted MONONINE solution to an administration set made with microbore tubing. Use of other administration sets without microbore tubing may result in a larger retention of the solution within the administration set.
- Administer MONONINE at room temperature and within 3 hours of reconstitution.
- After administration, any unused solution and the administration equipment should be discarded.
Rate of Administration
The rate of administration should be determined by the response and comfort of the patient; intravenous dosage administration rates of up to 225 IU/minute have been regularly tolerated without incident. When reconstituted as directed, i.e., to approximately 100 IU/mL, MONONINE should be administered at a rate of approximately 2.0 mL per minute.
More about Mononine (coagulation factor ix)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
Patient resources
Other brands
Idelvion, Alprolix, BeneFix, Rebinyn, ... +3 more
Professional resources
Other brands
Idelvion, Alprolix, BeneFix, Rebinyn, ... +3 more
Related treatment guides
See also:
Lysteda
Lysteda (tranexamic acid) is used to treat heavy menstrual bleeding. Includes Lysteda side effects ...
Cyklokapron
Cyklokapron is used for bleeding disorder, factor ix deficiency, hemophilia a
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Idelvion
Idelvion (coagulation factor ix recombinant, albumin fusion protein) is a long-acting, injectable ...
Alprolix
Alprolix is used in hemophilia B to help control and prevent bleeding, it is a coagulation Factor ...
BeneFix
BeneFix (coagulation factor IX) is a once-weekly, injectable, treatment for hemophilia B that may ...
Rebinyn
Rebinyn (glycopegylated coagulation factor IX [recombinant]) is an injectable, long-lasting, human ...
Alphanine SD
Alphanine SD is used for factor ix deficiency, hemophilia b
Profilnine
Profilnine is used for factor ix deficiency, factor vii deficiency, hemophilia a with inhibitors ...
Tranexamic acid
Tranexamic acid is used for bleeding disorder, factor ix deficiency, heavy menstrual bleeding ...
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.