Emend Dosage
Generic name: aprepitant 80mg
Dosage form: capsule, powder for oral suspension
Drug class: NK1 receptor antagonists
Medically reviewed by Drugs.com. Last updated on Dec 1, 2023.
Recommended Dosage
Adults and Pediatric Patients 12 Years of Age and Older
The recommended oral dosage of EMEND capsules, dexamethasone, and a 5-HT3 antagonist in adults and pediatric patients 12 years of age and older who can swallow oral capsules, for the prevention of nausea and vomiting associated with administration of HEC or MEC is shown in Table 1 or Table 2, respectively. For patients who cannot swallow oral capsules, EMEND for oral suspension can be used instead of EMEND capsules as shown in Table 3.
| Population | Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|---|
|
|||||
| EMEND capsules* | Adults and Pediatric Patients 12 Years and Older | 125 mg orally | 80 mg orally | 80 mg orally | none |
| Dexamethasone | Adults | 12 mg orally | 8 mg orally | 8 mg orally | 8 mg orally |
| Pediatric Patients 12 Years and Older | If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].† | ||||
| 5-HT3 antagonist | Adults and Pediatric Patients 12 Years and Older | See selected 5-HT3 antagonist prescribing information for the recommended dosage | none | none | none |
| Population | Day 1 | Day 2 | Day 3 | |
|---|---|---|---|---|
|
||||
| EMEND capsules* | Adults and Pediatric Patients 12 Years and Older | 125 mg orally | 80 mg orally | 80 mg orally |
| Dexamethasone | Adults | 12 mg orally | none | none |
| Pediatric Patients 12 Years and Older | If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].† | |||
| 5-HT3 antagonist | Adults and Pediatric Patients 12 Years and Older | See the selected 5-HT3 antagonist prescribing information for recommended dosage | none | none |
Pediatric Patients 6 Months to less than 12 Years of Age or Pediatric and Adult Patients Unable to Swallow Capsules
The recommended dose of EMEND for oral suspension to be administered with a 5-HT3 antagonist, with or without a corticosteroid, for the prevention of nausea and vomiting associated with administration of HEC or MEC is specified in Table 3. Dosing of EMEND for oral suspension is based on weight, to a maximum of 125 mg on Day 1 and 80 mg on Days 2 and 3. Dosing in pediatric patients less than 6 kg is not recommended.
| Population | Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|---|
|
|||||
| EMEND for oral suspension* | Pediatric Patients 6 Months to Less than12 Years or Pediatric and Adult Patients Unable to Swallow Capsules | 3 mg/kg orally Maximum dose 125 mg | 2 mg/kg orally Maximum dose 80 mg | 2 mg/kg orally Maximum dose 80 mg | none |
| Dexamethasone | Adults Unable to Swallow Capsules | See Table 1 or 2 | See Table 1 or 2 | See Table 1 or 2 | See Table 1 or 2 |
| Pediatric Patients 6 Months to Less than12 Years or Pediatric Patients Unable to Swallow Capsules | If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].† | ||||
| 5-HT3 antagonist | Pediatric Patients 6 Months to Less than12 Years or Pediatric and Adult Patients Unable to Swallow Capsules | See selected 5-HT3 antagonist prescribing information for the recommended dosage | none | none | none |
Preparation Instructions for EMEND for Oral Suspension -- for Healthcare Providers
EMEND for oral suspension should be prepared by a healthcare provider. Once prepared, it may be administered either by a healthcare provider, patient, or caregiver.
Before preparing EMEND:
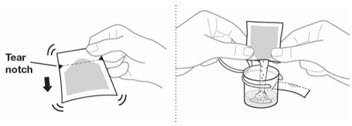
- Do not open the pouch of EMEND until ready to prepare the medicine.
- Store the pouch at room temperature [between 68°F-77°F (20°C-25°C)].
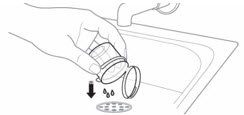
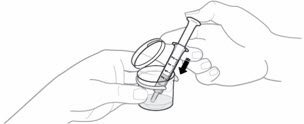
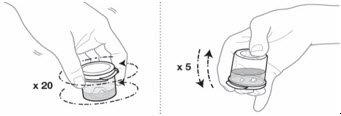
| EMEND for oral suspension is packaged as a kit with one 1 mL oral dosing dispenser, one 5 mL oral dosing dispenser, one cap and one mixing cup. | 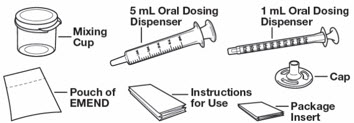 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
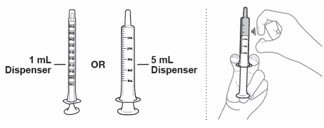 |
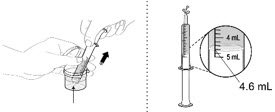
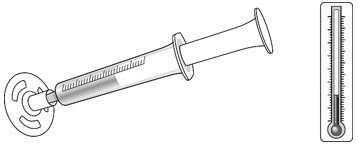
| Make sure no air is in the dispenser - if air is present, remove. Make sure the dispenser contains the prescribed dose. |
|
|
 |
|
Administration Instructions
EMEND capsules and EMEND for oral suspension can be administered with or without food.
EMEND for oral suspension
- The dose will be prepared by the healthcare provider and dispensed to the patient or caregiver in an oral dispenser.
- Keep the dispenser in the refrigerator until administered to the patient. The dose can be stored at room temperature for up to 3 hours before use.
- When ready to use, take the cap off the dispenser, place the dispenser in the patient's mouth along the inner cheek on either the right or left side. Slowly dispense the medicine.
- The dose must be used within 72 hours of preparation.
- Discard any doses remaining after 72 hours.
Frequently asked questions
More about Emend (aprepitant)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (16)
- Drug images
- Side effects
- During pregnancy
- Generic availability
- Drug class: NK1 receptor antagonists
- En español
Patient resources
Other brands
Professional resources
Other brands
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.