Decadron: Package Insert / Prescribing Info
Package insert / product label
Generic name: dexamethasone
Dosage form: tablet
Drug class: Glucocorticoids
Medically reviewed by Drugs.com. Last updated on Dec 23, 2024.
The Decadron brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Decadron Description
Decadron® Tablets 0.5 mg, 0.75 mg, 4 mg and 6 mg are for oral administration. Each tablet contains 0.5 mg, 0.75 mg, 4 mg or 6 mg of dexamethasone.
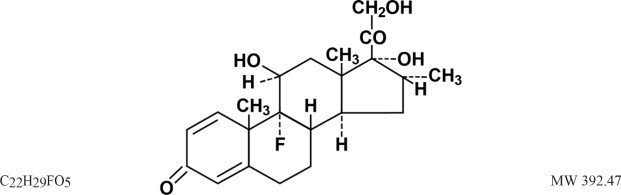
Dexamethasone, a synthetic adrenocortical steroid, is a white to practically white, odorless, crystalline powder. It is stable in air. It is practically insoluble in water. It is designated chemically as 9-fluoro-11β, 17, 21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione.
The structural formula is represented below:
Each tablet contains anhydrous lactose, croscarmellose sodium, magnesium stearate, microcrystalline cellulose and stearic acid. In addition, the 0.5 mg tablet contains D&C Yellow #10 and FD&C Yellow #5. The 0.75 mg tablet contains D&C Yellow #10 and FD&C Blue #1.
Decadron - Clinical Pharmacology
Glucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Glucocorticoids cause varied metabolic effects. In addition, they modify the body's immune responses to diverse stimuli. Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have sodium-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs including dexamethasone are primarily used for their anti-inflammatory effects in disorders of many organ systems.
At equipotent anti-inflammatory doses, dexamethasone almost completely lacks the sodium-retaining property of hydrocortisone and closely related derivatives of hydrocortisone.
Indications and Usage for Decadron
Allergic States
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity reactions, seasonal or perennial allergic rhinitis and serum sickness.
Dermatologic Diseases
Bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus, and severe erythema multiforme (Stevens-Johnson syndrome).
Endocrine Disorders
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; may be used in conjunction with synthetic mineralocorticoid analogs where applicable; in infancy mineralocorticoid supplementation is of particular importance), congenital adrenal hyperplasia, hypercalcemia associated with cancer, and nonsuppurative thyroiditis.
Gastrointestinal Diseases
To tide the patient over a critical period of the disease in regional enteritis and ulcerative colitis.
Hematologic Disorders
Acquired (autoimmune) hemolytic anemia, congenital (erythroid) hypoplastic anemia (Diamond-Blackfan anemia), idiopathic thrombocytopenic purpura in adults, pure red cell aplasia, and selected cases of secondary thrombocytopenia.
Miscellaneous
Diagnostic testing of adrenocortical hyperfunction, trichinosis with neurologic or myocardial involvement, tuberculous meningitis with subarachnoid block or impending block when used with appropriate antituberculous chemotherapy.
Nervous System
Acute exacerbations of multiple sclerosis, cerebral edema associated with primary or metastatic brain tumor, craniotomy, or head injury.
Ophthalmic Diseases
Sympathetic ophthalmia, temporal arteritis, uveitis, and ocular inflammatory conditions unresponsive to topical corticosteroids.
Renal Diseases
To induce a diuresis or remission of proteinuria in idiopathic nephrotic syndrome or that due to lupus erythematosus.
Respiratory Diseases
Berylliosis, fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis.
Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis, acute rheumatic carditis, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy). For the treatment of dermatomyositis, polymyositis, and systemic lupus erythematosus.
Contraindications
Systemic fungal infections (see WARNINGS: Fungal Infections). Dexamethasone tablets are contraindicated in patients who are hypersensitive to any components of this product.
Warnings
General:
Rare instances of anaphylactoid reactions have occurred in patients receiving corticosteroid therapy (see ADVERSE REACTIONS).
Increased dosage of rapidly acting corticosteroids is indicated in patients on corticosteroid therapy subjected to any unusual stress before, during, and after the stressful situation.
Cardio-Renal:
Average and large doses of corticosteroids can cause elevation of blood pressure, sodium and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Endocrine:
Corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for corticosteroid insufficiency after withdrawal of treatment. Adrenocortical insufficiency may result from too rapid withdrawal of corticosteroids and may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted.
If the patient is receiving steroids already, dosage may have to be increased.
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.
Infections
General:
Patients who are on corticosteroids are more susceptible to infections than healthy individuals. There may be decreased resistance and inability to localize infection when corticosteroids are used. Infection with any pathogen (viral, bacterial, fungal, protozoan or helminthic) in any location of the body may be associated with the use of corticosteroids alone or in combination with other immunosuppressive agents.
These infections may be mild to severe. With increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may also mask some signs of current infection.
Fungal Infections:
Corticosteroids may exacerbate systemic fungal infections and therefore should not be used in the presence of such infections unless they are needed to control life-threatening drug reactions. There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure (see PRECAUTIONS: Drug Interactions: Amphotericin B injection and potassium-depleting agents).
Special Pathogens:
Latent disease may be activated or there may be an exacerbation of intercurrent infections due to pathogens, including those caused by Amoeba, Candida, Cryptococcus, Mycobacterium, Nocardia, Pneumocystis, Toxoplasma.
It is recommended that latent amebiasis or active amebiasis be ruled out before initiating corticosteroid therapy in any patient who has spent time in the tropics or any patient with unexplained diarrhea.
Similarly, corticosteroids should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Corticosteroids should not be used in cerebral malaria.
Tuberculosis:
The use of corticosteroids in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculous regimen.
If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Vaccination:
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered. However, the response to such vaccines cannot be predicted. Immunization procedures may be undertaken in patients who are receiving corticosteroids as replacement therapy, e.g., for Addison's disease.
Viral infections:
Chickenpox and measles can have a more serious or even fatal course in pediatric and adult patients on corticosteroids. In pediatric and adult patients who have not had these diseases, particular care should be taken to avoid exposure. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with immune globulin (IG) may be indicated. (See the respective package inserts for VZIG and IG for complete prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Ophthalmic:
Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should not be used in active ocular herpes simplex.
Precautions
General:
The lowest possible dose of corticosteroids should be used to control the condition under treatment. When reduction in dosage is possible, the reduction should be gradual.
Since complications of treatment with corticosteroids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement.
Cardio-Renal:
As sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids, these agents should be used with caution in patients with congestive heart failure, hypertension, or renal insufficiency.
Endocrine:
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
Gastrointestinal:
Steroids should be used with caution in active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses, and nonspecific ulcerative colitis, since they may increase the risk of a perforation.
Signs of peritoneal irritation following gastrointestinal perforation in patients receiving corticosteroids may be minimal or absent.
There is an enhanced effect due to decreased metabolism of corticosteroid after withdrawal of treatment.
Musculoskeletal:
Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (i.e., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis (e.g., postmenopausal women) before initiating corticosteroid therapy.
Neuro-Psychiatric:
Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that they affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect. (See DOSAGE AND ADMINISTRATION.)
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Ophthalmic:
lntraocular pressure may become elevated in some individuals. If steroid therapy is continued for more than 6 weeks, intraocular pressure should be monitored.
Information for Patients
Patients should be warned not to discontinue the use of corticosteroids abruptly or without medical supervision. As prolonged use may cause adrenal insufficiency and make patients dependent on corticosteroids, they should advise any medical attendants that they are taking corticosteroids and they should seek medical advice at once should they develop an acute illness including fever or other signs of infection. Following prolonged therapy, withdrawal of corticosteroids may result in symptoms of the corticosteroid withdrawal syndrome including, myalgia, arthralgia, and malaise.
Persons who are on corticosteroids should be warned to avoid exposure to chickenpox or measles.
Patients should also be advised that if they are exposed, medical advice should be sought without delay.
Drug Interactions
Amphotericin B injection and potassium-depleting agents:
When corticosteroids are administered concomitantly with potassium-depleting agents (e.g., amphotericin B, diuretics), patients should be observed closely for development of hypokalemia. In addition, there have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure.
Antibiotics:
Macrolide antibiotics have been reported to cause a significant decrease in corticosteroid clearance (see Drug Interactions: Hepatic Enzyme Inducers, Inhibitors and Substrates).
Anticholinesterases:
Concomitant use of anticholinesterase agents and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy.
Anticoagulants, Oral
Co-administration of corticosteroids and warfarin usually results in inhibition of response to warfarin, although there have been some conflicting reports. Therefore, coagulation indices should be monitored frequently to maintain the desired anticoagulant effect.
Antidiabetics:
Because corticosteroids may increase blood glucose concentrations, dosage adjustments of antidiabetic agents may be required.
Cyclosporine:
Increased activity of both cyclosporine and corticosteroids may occur when the two are used concurrently. Convulsions have been reported with this concurrent use.
Dexamethasone Suppression Test (DST):
False-negative results in the dexamethasone suppression test (DST) in patients being treated with indomethacin have been reported. Thus, results of the DST should be interpreted with caution in these patients.
Digitalis Glycosides:
Patients on digitalis glycosides may be at increased risk of arrhythmias due to hypokalemia.
Ephedrine:
Ephedrine may enhance the metabolic clearance of corticosteroids, resulting in decreased blood levels and lessened physiologic activity, thus requiring an increase in corticosteroid dosage.
Estrogens, including Oral Contraceptives:
Estrogens may decrease the hepatic metabolism of certain corticosteroids, thereby increasing their effect.
Hepatic Enzyme Inducers, Inhibitors and Substrates:
Drugs which induce cytochrome P450 3A4 (CYP 3A4) enzyme activity (e.g., barbiturates, phenytoin, carbamazepine, rifampin) may enhance the metabolism of corticosteroids and require that the dosage of the corticosteroid be increased. Drugs which inhibit CYP 3A4 (e.g., ketoconazole, macrolide antibiotics such as erythromycin) have the potential to result in increased plasma concentrations of corticosteroids. Dexamethasone is a moderate inducer of CYP 3A4. Co-administration with other drugs that are metabolized by CYP 3A4 (e.g., indinavir, erythromycin) may increase their clearance, resulting in decreased plasma concentration.
Ketoconazole:
Ketoconazole has been reported to decrease the metabolism of certain corticosteroids by up to 60%, leading to increased risk of corticosteroid side effects. In addition, ketoconazole alone can inhibit adrenal corticosteroid synthesis and may cause adrenal insufficiency during corticosteroid withdrawal.
Nonsteroidal Anti-Inflammatory Agents (NSAIDS):
Concomitant use of aspirin (or other nonsteroidal anti-inflammatory agents) and corticosteroids increases the risk of gastrointestinal side effects. Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia. The clearance of salicylates may be increased with concurrent use of corticosteroids.
Phenytoin:
In post-marketing experience, there have been reports of both increases and decreases in phenytoin levels with dexamethasone co-administration, leading to alterations in seizure control.
Thalidomide:
Co-administration with thalidomide should be employed cautiously, as toxic epidermal necrolysis has been reported with concomitant use.
Vaccines:
Patients on corticosteroid therapy may exhibit a diminished response to toxoids and live or inactivated vaccines due to inhibition of antibody response. Corticosteroids may also potentiate the replication of some organisms contained in live attenuated vaccines. Routine administration of vaccines or toxoids should be deferred until corticosteroid therapy is discontinued if possible (see WARNINGS: Infections, Vaccination).
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether corticosteroids have a potential for carcinogenesis or mutagenesis.
Steroids may increase or decrease motility and number of spermatozoa in some patients.
Pregnancy
Teratogenic Effects: Pregnancy Category C
Corticosteroids have been shown to be teratogenic in many species when given in doses equivalent to the human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring. There are no adequate and well-controlled studies in pregnant women. Corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Nursing Mothers:
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Because of the potential for serious adverse reactions in nursing infants from corticosteroids, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use:
The efficacy and safety of corticosteroids in the pediatric population are based on the well-established course of effect of corticosteroids, which is similar in pediatric and adult populations. Published studies provide evidence of efficacy and safety in pediatric patients for the treatment of nephrotic syndrome (patients >2 years of age), and aggressive lymphomas and leukemias (patients >1 month of age). Other indications for pediatric use of corticosteroids, e.g., severe asthma and wheezing, are based on adequate and well-controlled trials conducted in adults, on the premises that the course of the diseases and their pathophysiology are considered to be substantially similar in both populations.
The adverse effects of corticosteroids in pediatric patients are similar to those in adults (see ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Pediatric patients who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression (i.e., cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The linear growth of pediatric patients treated with corticosteroids should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of treatment alternatives. In order to minimize the potential growth effects of corticosteroids, pediatric patients should be titrated to the lowest effective dose.
Geriatric Use:
Clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In particular, the increased risk of diabetes mellitus, fluid retention and hypertension in elderly patients treated with corticosteroids should be considered.
Adverse Reactions/Side Effects
(listed alphabetically, under each subsection)
The following adverse reactions have been reported with dexamethasone or other corticosteroids:
Cardiovascular:
Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction (see WARNINGS: Cardio-renal), edema, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis.
Dermatologic:
Acne, allergic dermatitis, dry scaly skin, ecchymoses and petechiae, erythema, impaired wound healing, increased sweating, rash, striae, suppression of reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria.
Endocrine:
Decreased carbohydrate and glucose tolerance, development of cushingoid state, hyperglycemia, glycosuria, hirsutism, hypertrichosis, increased requirements for insulin or oral hypoglycemic agents in diabetes, manifestations of latent diabetes mellitus, menstrual irregularities, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery, or illness), suppression of growth in pediatric patients.
Fluid and Electrolyte Disturbances:
Congestive heart failure in susceptible patients, fluid retention, hypokalemic alkalosis, potassium loss, sodium retention.
Gastrointestinal:
Abdominal distention, elevation in serum liver enzyme levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, perforation of the small and large bowel (particularly in patients with inflammatory bowel disease), ulcerative esophagitis.
Musculoskeletal:
Aseptic necrosis of femoral and humeral heads, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, steroid myopathy, tendon rupture, vertebral compression fractures.
Neurological/Psychiatric:
Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually after treatment, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychic disorders, vertigo.
Related/similar drugs
Overdosage
Treatment of overdosage is by supportive and symptomatic therapy. In the case of acute overdosage, according to the patient's condition, supportive therapy may include gastric lavage or emesis.
Decadron Dosage and Administration
For Oral Administration:
The initial dose varies from 0.75 to 9 mg a day depending on the disease being treated.
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.
After a favorable initial response is noted, the proper maintenance dosage should be determined by decreasing the initial dosage in small decrements at appropriate time intervals until the lowest dosage that maintains an adequate clinical response is reached.
Situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient's individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment. In this latter situation it may be necessary to increase the dosage of the corticosteroid for a period of time consistent with the patient's condition. If after long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
In the treatment of acute exacerbations of multiple sclerosis, daily doses of 30 mg of dexamethasone for a week followed by 4 to 12 mg every other day for one month have been shown to be effective (see PRECAUTIONS: Neuro-Psychiatric).
In pediatric patients, the initial dose of dexamethasone may vary depending on the specific disease entity being treated. The range of initial doses is 0.02 to 0.3 mg/kg/day in three or four divided doses (0.6 to 9 mg/m2bsa/day).
For the purpose of comparison, the following is the equivalent milligram dosage of the various corticosteroids:
| Cortisone, 25 | Triamcinolone, 4 | |
| Hydrocortisone, 20 | Paramethasone, 2 | |
| Prednisolone, 5 | Betamethasone, 0.75 | |
| Prednisone, 5 | Dexamethasone, 0.75 | |
| Methylprednisolone, 4 |
These dose relationships apply only to oral or intravenous administration of these compounds. When these substances or their derivatives are injected intramuscularly or into joint spaces, their relative properties may be greatly altered.
In acute, self-limited allergic disorders or acute exacerbations of chronic allergic disorders, the following dosage schedule combining parenteral and oral therapy is suggested:
Dexamethasone Sodium Phosphate injection, 4 mg per mL:
First Day
- 1 or 2 mL, intramuscularly
Dexamethasone tablets, 0.75 mg:
Second Day
- 4 tablets in two divided doses
Third Day
- 4 tablets in two divided doses
Fourth Day
- 2 tablets in two divided doses
Fifth Day
- 1 tablet
Sixth Day
- 1 tablet
Seventh Day
- No treatment
Eighth Day
- Follow-up visit
This schedule is designed to ensure adequate therapy during acute episodes, while minimizing the risk of overdosage in chronic cases.
In cerebral edema, dexamethasone sodium phosphate injection is generally administered initially in a dosage of 10 mg intravenously followed by 4 mg every six hours intramuscularly until the symptoms of cerebral edema subside. Response is usually noted within 12 to 24 hours and dosage may be reduced after two to four days and gradually discontinued over a period of five to seven days. For palliative management of patients with recurrent or inoperable brain tumors, maintenance therapy with either dexamethasone sodium phosphate injection or dexamethasone tablets in a dosage of 2 mg two or three times daily may be effective.
Dexamethasone Suppression Tests
- Tests for Cushing's syndrome
Give 1.0 mg of dexamethasone orally at 11:00 p.m. Blood is drawn for plasma cortisol determination at 8:00 a.m. the following morning.
For greater accuracy, give 0.5 mg of dexamethasone orally every 6 hours for 48 hours. Twenty-four hour urine collections are made for determination of 17-hydroxycorticosteroid excretion. - Test to distinguish Cushing's syndrome due to pituitary ACTH excess from Cushing's syndrome due to other causes.
Give 2.0 mg of dexamethasone orally every 6 hours for 48 hours. Twenty-four hour urine collections are made for determination of 17-hydroxycorticosteroid excretion.
How is Decadron supplied
Decadron® tablets are available as:
0.5 mg tablets scored (yellow), debossed “Par-084” and supplied in bottles of 100, NDC 58463-014-01.
0.75 mg tablets scored (blue), debossed “Par-085” and supplied in bottles of 100, NDC 58463-015-01.
4 mg tablets scored (white), debossed “Par-087” and supplied in bottles of 100, NDC 58463-016-01.
6 mg tablets scored (white), debossed “Par-129” and supplied in bottles of 100, NDC 58463-017-01.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light resistant container as defined in the USP/NF.
Distributed by:
Fera Pharmaceuticals, LLC
for Pragma Pharmaceuticals, LLC
Locust Valley, N.Y. 11560
Pragma®
PPI-014 Revised: 01/18
Decadron® is a registered trademark of Pragma Pharmaceuticals, LLC
Principal Display Panel - Decadron Tablets 0.5 mg
NDC 58463-014-01
DECADRON®
(dexamethasone
tablets, USP)
0.5 mg
100 Tablets
Rx only
Pragma®
Principal Display Panel - Decadron Tablets 0.75 mg
NDC 58463-015-01
DECADRON®
(dexamethasone
tablets, USP)
0.75 mg
100 Tablets
Rx only
Pragma®
| DECADRON
dexamethasone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DECADRON
dexamethasone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DECADRON
dexamethasone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DECADRON
dexamethasone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pragma Pharmaceuticals, LLC (078813515) |
Frequently asked questions
- How long does dexamethasone stay in your system?
- What is a dexamethasone suppression test used for?
- Can I use expired neomycin and polymyxin b sulfates, dexamethasone ophthalmic?
More about Decadron (dexamethasone)
- Check interactions
- Compare alternatives
- Reviews (29)
- Drug images
- Latest FDA alerts (9)
- Side effects
- Dosage information
- During pregnancy
- Drug class: glucocorticoids
- Breastfeeding
Patient resources
Professional resources
Other brands
Dxevo, Zcort, Dexpak Taperpak, HiDex, ... +6 more