Benzocaine Gel Prescribing Information
Package insert / product label
Dosage form: gel, dentifrice
Drug class: Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Warnings
Keep out of reach of children. Do not use on people with known allergies to benzocaine and PABA compounds.
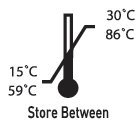
Benzocaine Gel Description
Each gram of Darby 20% Benzocaine Gel contains between 180-220 mg benzocaine U.S.P. in a water soluble base of PEG 3350 U.S.P., PEG 400 U.S.P., flavor, sodium saccharin U.S.P., FD&C Red #40
Warnings
Keep out of reach of children. Do not use on people with known allergies to benzocaine and PABA compounds.

Benzocaine Gel Description
Each gram of Darby 20% Benzocaine Gel contains between 180-220 mg benzocaine U.S.P. in a water soluble base of PEG 3350 U.S.P., PEG 400 U.S.P., flavor, sodium saccharin U.S.P., FD&C Green #5
Warnings
Keep out of reach of children. Do not use on people with known allergies to benzocaine and PABA compounds.

Benzocaine Gel Description
Each gram of Darby 20% Benzocaine Gel contains between 180-220 mg benzocaine U.S.P. in a water soluble base of PEG 3350 U.S.P., PEG 400 U.S.P., flavor, sodium saccharin U.S.P., FD&C Red #28
Warnings
Keep out of reach of children. Do not use on people with known allergies to benzocaine and PABA compounds.

Benzocaine Gel Description
Each gram of Darby 20% Benzocaine Gel contains between 180-220 mg benzocaine U.S.P. in a water soluble base of PEG 3350 U.S.P., PEG 400 U.S.P., flavor, sodium saccharin U.S.P., FD&C Red #40
Warnings
Keep out of reach of children. Do not use on people with known allergies to benzocaine and PABA compounds.

Benzocaine Gel Description
Each gram of Darby 20% Benzocaine Gel contains between 180-220 mg benzocaine U.S.P. in a water soluble base of PEG 3350 U.S.P., PEG 400 U.S.P., flavor, sodium saccharin U.S.P., FD&C Red #40, FD&C Blue #1 and FD&C Red #28
Warnings
Keep out of reach of children. Do not use on people with known allergies to benzocaine and PABA compounds.

Benzocaine Gel Description
Each gram of Darby 20% Benzocaine Gel contains between 180-220 mg benzocaine U.S.P. in a water soluble base of PEG 3350 U.S.P., PEG 400 U.S.P., flavor, sodium saccharin U.S.P.
PRINCIPAL DISPLAY PANEL - 34 g Jar Label (Cherry)
DARBY®
DARBY DENTAL SUPPLY, LLC
DARBY
QUALITY
CERTIFIED
9501737
NDC 6646730021
20% Benzocaine Gel
Topical Anesthetic Gel
Cherry Flavored | Net Contents: 1oz
Rx only
To reorder:
800-645-2310 or darby.com
Distributed by:
Darby Dental Supply, LLC, Jericho, NY 11753

PRINCIPAL DISPLAY PANEL - 34 g Jar Label (Mint)
DARBY®
DARBY DENTAL SUPPLY, LLC
DARBY
QUALITY
CERTIFIED
9501738
NDC 6646730031
20% Benzocaine Gel
Topical Anesthetic Gel
Mint Flavored | Net Contents: 1oz
Rx only
To reorder:
800-645-2310 or darby.com
Distributed by:
Darby Dental Supply, LLC, Jericho, NY 11753

PRINCIPAL DISPLAY PANEL - 34 g Jar Label (Bubble Gum)
DARBY®
DARBY DENTAL SUPPLY, LLC
DARBY
QUALITY
CERTIFIED
9501736
NDC 6646730011
20% Benzocaine Gel
Topical Anesthetic Gel
Bubble Gum Flavored | Net Contents: 1oz
Rx only
To reorder:
800-645-2310 or darby.com
Distributed by:
Darby Dental Supply, LLC, Jericho, NY 11753

PRINCIPAL DISPLAY PANEL - 34 g Jar Label (Strawberry)
DARBY®
DARBY DENTAL SUPPLY, LLC
DARBY
QUALITY
CERTIFIED
9501744
NDC 6646730061
20% Benzocaine Gel
Topical Anesthetic Gel
Strawberry Flavored | Net Contents: 1oz
Rx only
To reorder:
800-645-2310 or darby.com
Distributed by:
Darby Dental Supply, LLC, Jericho, NY 11753

PRINCIPAL DISPLAY PANEL - 34 g Jar Label (Raspberry)
DARBY®
DARBY DENTAL SUPPLY, LLC
DARBY
QUALITY
CERTIFIED
9501734
NDC 6646730001
20% Benzocaine Gel
Topical Anesthetic Gel
Raspberry Flavored | Net Contents: 1oz
Rx only
To reorder:
800-645-2310 or darby.com
Distributed by:
Darby Dental Supply, LLC, Jericho, NY 11753

PRINCIPAL DISPLAY PANEL - 34 g Jar Label (Pina Colada)
DARBY®
DARBY DENTAL SUPPLY, LLC
DARBY
QUALITY
CERTIFIED
9501739
NDC 6646730041
20% Benzocaine Gel
Topical Anesthetic Gel
Piña Colada Flavored | Net Contents: 1oz
Rx only
To reorder:
800-645-2310 or darby.com
Distributed by:
Darby Dental Supply, LLC, Jericho, NY 11753

| BENZOCAINE
CHERRY
benzocaine gel, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| BENZOCAINE
MINT
benzocaine gel, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| BENZOCAINE
BUBBLE GUM
benzocaine gel, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| BENZOCAINE
STRAWBERRY
benzocaine gel, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| BENZOCAINE
RASPBERRY
benzocaine gel, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| BENZOCAINE
PINA COLADA
benzocaine gel, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Darby Dental Supply, LLC (825137818) |
| Registrant - DSHealthcare (056296981) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DENTSPLY Caulk | 083235549 | MANUFACTURE(66467-3000, 66467-3001, 66467-3002, 66467-3003, 66467-3004, 66467-3006) | |
More about benzocaine topical
- Check interactions
- Compare alternatives
- Reviews (39)
- Latest FDA alerts (5)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical anesthetics
- Breastfeeding
Patient resources
Professional resources
Other brands
BeeGentle, Benzo-Jel, Denti-Care Denti-Freeze, Precaine B, ... +2 more
