Zuranolone
Generic name: Zuranolone
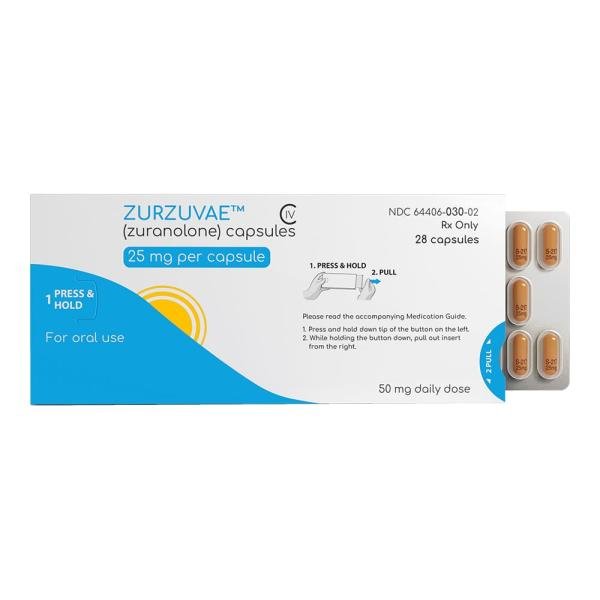
Brand name: Zurzuvae
Dosage form: capsules (20mg, 25mg, 30 mg)
Drug class: Miscellaneous antidepressants
What is Zuranolone?
Zuranolone capsules are used for the treatment postpartum depression (PPD), it is a rapid-acting, once-daily capsule taken for 14 days. Zuranolone works quickly to improve depression symptoms, starting in 3 days compared to current treatment options, which may take weeks or months to work. Zuranolone is the first oral medication to be approved by the FDA to treat postpartum depression.
Zurzuvae is the brand name for zuranolone. During development, it was called SAGE-217 and BIIB125.
Postpartum depression is a major depressive episode that can occur after childbirth but can also start during the later stages of pregnancy. PPD is a serious condition in which women may feel intense anxiety, sadness, shame, guilt, problems sleeping, stress, panic attacks, and suicidal thoughts or attempts. This can make it difficult for mothers to care for and bond with their babies and may delay the child's physical and emotional development.
Is Zuranolone FDA approved?
Yes, under the brand name Zurzuvae, FDA approval was granted for Zurzuvae to treat adults with postpartum depression on August 04, 2023. Approval was granted to Sage Therapeutics, Inc. The application for FDA approval was supported by the LANDSCAPE and NEST clinical trials.
How does Zuranolone work?
Zuranolone is a neuroactive steroid (NAS) GABA-A receptor positive allosteric modulator (PAM).
Zuranolone is thought to work by rebalancing brain networks responsible for functions such as mood, arousal, behavior, and cognition. Some health conditions, such as depression, are thought to be due to imbalances in GABA (Gamma-aminobutyric acid). GABA is a chemical messenger (neurotransmitter) in the brain and spinal cord (central nervous system). Zuranolone is a neuroactive steroid that acts on the GABA-A receptors as a positive allosteric modulator.
Zuranolone was developed as an improvement to brexanolone (Zulresso), which is only available as an intravenous injection given over 60 hours (2.5 days), whereas Zuranolone can be taken as a once-daily capsule.
How effective is Zuranolone?
A clinical trial called Study 1 (NCT04442503) looked into the efficacy of Zuranolone 50mg for treating postpartum depression in adults. The study found that women who took Zuranolone 50mg daily for 2 weeks had a statistically significant reduction in their depressive symptoms by day 15. The symptoms were measured using the 17-item Hamilton Rating Scale for Depression (HAMD-17).
- Zuranolone 50mg reduced the HAMD-17 score by −15.6 compared to the placebo score, which decreased by −11.6, a difference of -4.0.
- Symptoms of depression improved from day 3 and lasted through to day 42.
Zuranolone Side Effects
Zuranolone may cause serious side effects, including:
Increased risk of suicidal thoughts or actions. Zuranolone, along with other antidepressant medications, may increase the risk of suicidal thoughts and actions in people that are 24 years of age and younger. This medicine should only be used in adults. Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
It is important to watch for and try to prevent suicidal thoughts and actions.
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- Tell your healthcare provider right away if you have any new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Tell your healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you: attempts to commit suicide, panic attacks, thoughts about suicide or dying, new or worse irritability, new or worse depression, acting aggressive or being angry or violent, feeling very agitated or restless, an extreme increase in activity and talking, trouble sleeping, acting on dangerous impulses, new or worse anxiety, other unusual changes in behavior or mood.
Common Zuranolone side effects may include:
- Sleepiness or drowsiness
- Tiredness
- Dizziness
- Common cold
- Diarrhea
- Urinary tract infection.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Warnings
Decreased ability to drive or do other dangerous activities.
Zuranolone may decrease your awareness and alertness, which can affect your ability to drive safely or operate machinery, or do other dangerous activities. Do not drive, operate machinery, or do other dangerous activities until at least 12 hours after taking each capsule during your 14-day treatment with this medicine. You may not be able to tell on your own how much this medicine is affecting or whether you can drive safely.
Decreased awareness and alertness
Zuranolone may cause sleepiness, drowsiness, slow thinking, dizziness, confusion, and trouble walking therefore, you may be at a higher risk for falls while taking this medicine.
Drinking alcohol, other medicines that cause CNS depressant effects, or opioids while taking this medicine can make these symptoms worse and may also cause trouble breathing.
Tell your healthcare provider if you develop any of these symptoms or if they get worse during treatment with this medicine.
Your healthcare provider may lower your dose or stop treatment if you develop these symptoms.
Before taking this medicine
Tell your healthcare provider about all of your medical conditions, including if you:
- drink alcohol
- have abused or been dependent on prescription medicines, street drugs, or alcohol
- have kidney or liver problems.
Pregnancy
Tell your health care provider if you are pregnant or plan to become pregnant, as this medicine may harm your unborn baby.
If you are able to become pregnant, then while taking this medicine and for one week afterward you should use effective contraception.
You should tell your healthcare provider right away if you become pregnant while on Zuranolone.
There is a pregnancy registry for females who are take Zuranolone during their pregnancy. The purpose of the registry is to collect information about the health of females exposed to this medicine and their baby. If you become pregnant during treatment with this medicine, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visit online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/ .
Breastfeeding
Zuranolone passes into breast milk, and it is not known if it can harm your baby. Talk to your healthcare provider about the risks and benefits of breastfeeding and about the best way to feed your baby during treatment with this medicine.
Dosing information
Zuranolone is taken once a day in the evening, with fatty food, for 14 days (two weeks).
Dose: 50 mg evening.
Duration: 14 days.
Comments: Patients unable to tolerate 50 mg once daily may have dose reduced to 40 mg.
This medicine can be used as a single therapy or together with other oral antidepressant medications.
Zurzuvae is available as 20mg, 25mg and 30mg capsules.
What happens if I miss a dose?
If you forget to taking your dose, skip the missed dose and take the next dose at your regular time the next evening. Do not take extra capsules to make up for the missed dose. Continue taking Zuranolone once daily until you complete the rest of your treatment course.
What happens if I overdose?
If you take too much Zuranolone, it is very important to call your healthcare provider or Poison Help Line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What to avoid
You should not drive a car, operate machinery, or do other dangerous activities until at least 12 hours after taking each dose of this medicine, as Zuranolone may make you feel sleepy, confused, or dizzy.
You should not drink alcohol or take other medicines that make you sleepy or dizzy while taking this medicine without talking to your healthcare provider.
What other drugs will affect Zuranolone?
Drugs may interact with Zuranolone, this includes prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here. Tell your doctor about all your current medicines and any medicine you start or stop using.
It is especially important to tell your healthcare provider if you take:
- antidepressants
- opioids
- CNS depressants such as benzodiazepines.
Ingredients
Active ingredient: zuranolone
Zurzuvae Inactive ingredients: Capsules contain colloidal silicon dioxide, croscarmellose sodium, mannitol, microcrystalline cellulose, and sodium stearyl fumarate.
The capsule shells contain gelatin, red iron oxide, titanium dioxide, and yellow iron oxide. The imprinting ink contains ammonium hydroxide, black iron oxide, propylene glycol, and shellac glaze.
Storage
- Store at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep this medicine and all medicines out of the reach of children.
Company information
Zurzuvae Brand:
Manufactured for: Biogen Inc., 225 Binney Street, Cambridge, MA 02142
Registered trademark: Zurzuvae is a registered trademark of Sage Therapeutics, Inc.
References
- Effect of Zuranolone vs Placebo in Postpartum Depression
- Food and Drug Administration (FDA) Zurzuvae Product Label
- Biogen and Sage Therapeutics Announce FDA Accepts Filing of New Drug Application and Grants Priority Review of Zuranolone in the Treatment of Major Depressive Disorder and Postpartum Depression
- FDA Approves First Oral Treatment for Postpartum Depression
More about zuranolone
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous antidepressants
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.