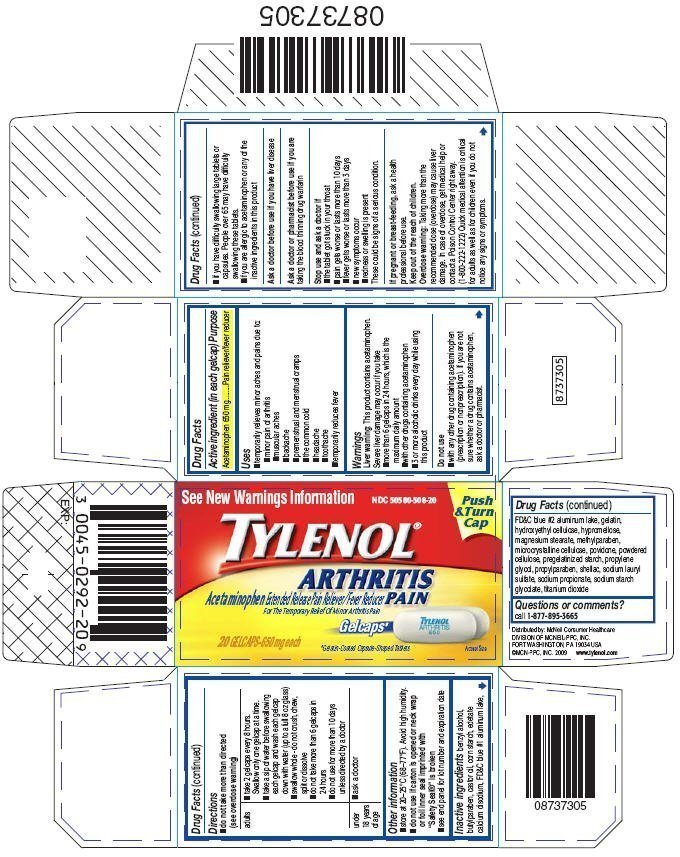
Tylenol Arthritis Pain Prescribing Information
Package insert / product label
Generic name: acetaminophen
Dosage form: tablet, film coated, extended release
Drug class: Miscellaneous analgesics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
Indications and Usage for Tylenol Arthritis Pain
- temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 gelcaps in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have difficulty swallowing large tablets or capsules. People over 65 may have difficulty swallowing these tablets.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Stop use and ask a doctor if
- the tablet got stuck in your throat
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Overdose warning
Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Tylenol Arthritis Pain Dosage and Administration
- do not take more than directed (see overdose warning)
| adults |
|
| under 18 years of age |
|
Storage and Handling
- store at 20–25°C (68–77°F). Avoid high humidity.
- do not use if carton is opened or neck wrap or foil inner seal imprinted with "Safety Seal®" is broken
- see end panel for lot number and expiration date
Inactive ingredients
benzyl alcohol, butylparaben, castor oil, corn starch, edetate calcium disodium, FD&C blue #1 aluminum lake, FD&C blue #2 aluminum lake, gelatin, hydroxyethyl cellulose, hypromellose, magnesium stearate, methylparaben, microcrystalline cellulose, povidone, powdered cellulose, pregelatinized starch, propylene glycol, propylparaben, shellac, sodium lauryl sulfate, sodium propionate, sodium starch glycolate, titanium dioxide
| TYLENOL
ARTHRITIS PAIN
acetaminophen tablet, film coated, extended release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - McNeil Consumer Healthcare Div McNeil-PPC, Inc (878046358) |
Frequently asked questions
- Can you take tramadol with acetaminophen, ibuprofen, or aspirin?
- What's the best sore throat medicine to use?
- What is paracetamol / Panadol called in the US?
- Acetaminophen vs Ibuprofen: Which is better?
- What medications cause liver enzymes to be elevated?
- Advil (ibuprofen) & Tylenol (acetaminophen) together, is it safe?
- How long does Percocet stay in your system?
- Does Mucinex help with Covid?
- What temperature is considered a fever?
More about Tylenol Arthritis Pain (acetaminophen)
- Check interactions
- Compare alternatives
- Reviews (6)
- Drug images
- Latest FDA alerts (19)
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous analgesics
- Breastfeeding
Patient resources
Professional resources
Other brands
Ofirmev, 7T Gummy ES Chewable Tablets