Sodium Chloride Irrigant: Package Insert / Prescribing Info
Package insert / product label
Dosage form: irrigation solution
Drug class: Miscellaneous topical agents
Medically reviewed by Drugs.com. Last updated on Sep 10, 2024.
Important Prescribing Information
September 3, 2024
| Subject: Temporary distribution of Sterile water and 0.9% Sodium chloride Semi-Rigid Bottle (SRB) Irrigation with non-U.S. labeling to address drug shortages |
Dear Healthcare Professional,
In order to address shortages of critical irrigation drug products, ICU Medical is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of products from our portfolio which are manufactured and intended for the Canadian market. Temporarily and effective immediately, ICU Medical will make available for sale in the US the products listed below. These products are manufactured in the same facility and to the same specifications as ICU Medical's FDA-approved drug products; however, they are not labeled with current FDA-approved labeling. The information contained in this letter pertains only to the products listed below, which are not approved by FDA.
| Product Description | Size | Canadian DIN | NDC |
|---|---|---|---|
| Sterile Water for Irrigation, USP | 1000 mL | DIN 00038229 | 0990-8139-10 |
| 0.9% Sodium Chloride Irrigation, USP | 250 mL | DIN 00037834 | 0990-5138-25 |
| 0.9% Sodium Chloride Irrigation, USP | 500 mL | DIN 00037834 | 0990-5138-50 |
| 0.9% Sodium Chloride Irrigation, USP | 1000 mL | DIN 00037834 | 0990-8138-10 |
There are some key differences in the labeling between the U.S. FDA-approved products and the Canadian products. Please see the product comparison tables at the end of this letter for additional information.
The barcode may not register accurately on the U.S. scanning systems. Institutions should manually input the product into their systems to confirm that barcode systems provide correct information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
The U.S. FDA-approved products are available by prescription only in the U.S. However, the Canadian products do not have the statement "Rx only" on their labeling.
Please refer to the FDA-approved package insert for the full prescribing information of each drug product.
If you have any questions about ordering SRB irrigation products, please contact ICU Medical Customer Care at (877) 946-7747 or email uscustomercare@icumed.com. For medical questions, please call ICU Medical Information at (800) 241-4002 or email Med-info_US@icumed.com.
To report adverse events or quality problems experienced with the use of this product, please call ICU Medical Global Complaint Management at (844) 654-7780 or email ProductComplaintsPP@icumed.com.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA's MedWatch Adverse Event Reporting Program either online, or regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call (800) 332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to (800) FDA-0178 (1-800-332-0178).
Our team is working with customers to mitigate the impact of this shortage and are committed to returning to full levels of production as soon as possible.
Sincerely,

Stuart Green
Senior Director, Quality
Representative Product Labels Comparison Tables
Sterile Water for Irrigation, USP
| US FDA Approved Product | Canadian Approved Product | |
 |  |
|
| Product Name | Sterile Water for Irrigation, USP | Sterile Water for Irrigation, USP |
| Indications | For irrigation only | For intraperitoneal, irrigation and topical use only |
| Active Ingredients | Sterile Water | Sterile Water |
| Additional Information | pH 5.5 (5.0 – 7.0) | pH approx. 5.5 |
| Storage Conditions | Store at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature] | 20 to 25°C |
| Container Type | AQUALITE™ Semi-Rigid Bottle | AQUALITE™ Semi-Rigid Bottle |
| Language | English | English and French |
| Additional Product Identifier | NDC number | DIN and product catalog number |
| Barcode | GS1 Primary Data Structure Includes product NDC Number | GS1 Primary Data Structure Includes Canada Manufacturer Number and unique product code |
0.9% Sodium Chloride Irrigation, USP
| US FDA Approved Product | Canadian Approved Product | |
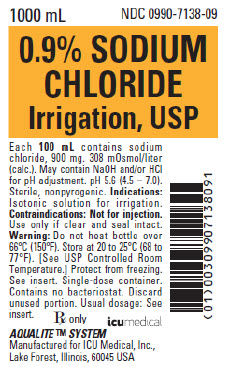 |  |
|
| Product Name | 0.9% Sodium Chloride Irrigation, USP | 0.9% Sodium Chloride Irrigation, USP |
| Indications | Isotonic solution for irrigation | For intraperitoneal, irrigation and topical use only |
| Active Ingredients | Each 100 mL contains sodium chloride, 900 mg | Each mL contains: Sodium chloride 9 mg |
| Additional Information | pH 5.6 (4.5 – 7.0) | pH approx. 5.6 |
| Storage Conditions | Store at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature] | 20 to 25°C |
| Container Type | AQUALITE™ Semi-Rigid Bottle | AQUALITE™ Semi-Rigid Bottle |
| Language | English | English and French |
| Additional Product Identifier | NDC number | DIN and product catalog number |
| Barcode | GS1 Primary Data Structure Includes product NDC Number | GS1 Primary Data Structure Includes Canada Manufacturer Number and unique product code |
PRINCIPAL DISPLAY PANEL - 250 mL Bottle Label
250 mL
Sterile Solution
DIN 00037834
N° 06138125
AQUALITE™
0.9%
Sodium Chloride
Irrigation USP
For Intraperitoneal, Irrigation and Topical
Use • NOT FOR INJECTION • Nonpyrogenic
• Single-Use
Use only if solution is clear and seal intact.
Discard unused portion. Each mL contains:
Sodium chloride 9 mg. May contain NaOH/HCl for
pH adjustment. Contains no bacteriostat. Dosage:
As directed. Do not heat over 66°C. Storage: 20 to
25°C. Protect from freezing.
pH approx. 5.6
Questions or Concerns
ICU Medical Canada Inc.
Saint-Laurent, QC H4S 0A9
RL-7298
LOT
EXP

PRINCIPAL DISPLAY PANEL - 500 mL Bottle Label
500 mL
Sterile Solution
DIN 00037834
N° 06138023
AQUALITE™
0.9%
Sodium Chloride Irrigation USP
For Intraperitoneal, Irrigation and Topical Use
• NOT FOR INJECTION • Nonpyrogenic • Single-Use
Use only if solution is clear and seal
intact. Discard unused portion. Each
mL contains: Sodium chloride 9 mg.
May contain NaOH and/or HCl for pH
adjustment. Contains no bacteriostat.
Dosage: As directed. Do not heat over
66°C. Storage: 20 to 25°C. Protect from
freezing.
pH approx. 5.6
Questions or Concerns
ICU Medical Canada Inc.
Saint-Laurent, QC H4S 0A9
icumedical
RL-7297
LOT
EXP

PRINCIPAL DISPLAY PANEL - 1000 mL Bottle Label
1000 mL
AQUALITE™
DIN 00037834
N° 07138019
0.9%
Sodium Chloride
Irrigation USP
For Intraperitoneal, Irrigation and Topical Use
•NOT FOR INJECTION
Sterile Solution • Nonpyrogenic • Single-Dose
Use only if solution is clear and seal intact.
Discard unused portion. Each mL contains:
Sodium chloride 9 mg. May contain NaOH/HCl
for pH adjustment. Contains no bacteriostat.
Usual Dose: As directed. Do not heat
container over 66°C. Storage: 20 to 25°C.
Protect from freezing.
pH approx. 5.6
Questions or Concerns
ICU Medical Canada Inc.
Saint-Laurent, QC H4S 0A9
icumedical
RL-7286
LOT
EXP

| SODIUM CHLORIDE
sodium chloride irrigant |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride irrigant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ICU Medical Inc. (118380146) |
