Sodium Chloride Irrigation: Package Insert / Prescribing Info
Package insert / product label
Dosage form: irrigation
Drug class: Miscellaneous topical agents
Medically reviewed by Drugs.com. Last updated on Sep 16, 2023.
On This Page
Package Insert
PIC™ (Plastic Irrigation Container)
0.9% Sodium Chloride Irrigation USP
Isotonic Solution for Irrigation.
For Irrigation Only.
Not for Injection.
Rx only
Sodium Chloride Irrigation Description
Each 100 mL contains:
Sodium Chloride USP 0.9 g; Water for Injection USP qs
pH adjusted with Hydrochloric Acid NF
pH: 5.0 (4.5–7.0) Calculated Osmolarity: 310 mOsmol/liter
Concentration of Electrolytes (mEq/liter): Sodium 154; Chloride 154
0.9% Sodium Chloride Irrigation USP is sterile, nonpyrogenic, isotonic and contains no bacteriostatic or antimicrobial agents.
The formula of the active ingredient is:
| Ingredient | Molecular Formula | Molecular Weight |
|---|---|---|
| Sodium Chloride USP | NaCl | 58.44 |
The plastic container is a copolymer of ethylene and propylene formulated and developed for parenteral drugs. The copolymer contains no plasticizers and exhibits virtually no leachability. The plastic container is also virtually impermeable to vapor transmission and, therefore, requires no overwrap to maintain the proper drug concentration. The safety of the plastic container has been confirmed by biological evaluation procedures. The material passes Class Vl testing as specified in the U.S. Pharmacopeia for Biological Tests — Plastic Containers. These tests have shown that the container is nontoxic and biologically inert.
Not made with natural rubber latex, PVC or DEHP.
Sodium Chloride Irrigation - Clinical Pharmacology
0.9% Sodium Chloride Irrigation USP is utilized for a variety of clinical indications such as sterile irrigation of body cavities, tissues or wounds, indwelling urethral catheters, surgical drainage tubes, and for washing, rinsing or soaking surgical dressings, instruments and laboratory specimens. It also serves as a diluent or vehicle for drugs used for irrigation or other pharmaceutical preparations.
0.9% Sodium Chloride Irrigation USP provides an isotonic saline irrigation identical in composition with 0.9% Sodium Chloride Injection USP (normal saline).
Physiological irrigation solutions are considered generally compatible with living tissues and organs.
Sodium, the major cation of the extracellular fluid, functions primarily in the control of water distribution, fluid balance, and osmotic pressure of body fluids. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid.
Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration.
Indications and Usage for Sodium Chloride Irrigation
0.9% Sodium Chloride Irrigation USP is indicated for all general irrigation, washing, rinsing and dilution purposes which permit use of a sterile, nonpyrogenic electrolyte solution.
Contraindications
0.9% Sodium Chloride Irrigation USP is not for injection by usual parenteral routes.
An electrolyte solution should not be used for irrigation during electrosurgical procedures.
Warnings
FOR IRRIGATION ONLY. NOT FOR INJECTION.
Irrigating fluids have been demonstrated to enter the systemic circulation in relatively large volumes; thus, irrigation solutions must be regarded as systemic drugs. Absorption of large amounts can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of the administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration.
Do not warm above 150°F (66°C).
After opening container, its contents should be used promptly to minimize the possibility of bacterial growth or pyrogen formation.
Discard unused portion of irrigating solution since it contains no preservatives.
Precautions
General
Use aseptic technique when preparing and administering sterile irrigation solutions.
Use only if solution is clear and container and seal are intact.
Do not use for irrigation that may result in absorption of large amounts of fluid into the blood.
Caution should be observed when the solution is used for continuous irrigation or allowed to “dwell” inside body cavities because of possible absorption into the blood stream and the production of circulatory overload.
When used for irrigation via appropriate irrigation equipment, the administration set should be attached promptly. Unused portions should be discarded and a fresh container of appropriate size used for the start up of each cycle or repeat procedure. For repeated irrigations of urethral catheters, a separate container should be used for each patient.
Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance after prolonged irrigation, when fluid absorption is suspected, or whenever the condition of the patient warrants such evaluation.
Drug Interactions
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic technique. Mix thoroughly. Do not store.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with 0.9% Sodium Chloride Irrigation USP have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with 0.9% Sodium Chloride Irrigation USP. It is also not known whether 0.9% Sodium Chloride Irrigation USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 0.9% Sodium Chloride Irrigation USP should be given to a pregnant woman only if clearly needed.
Labor and Delivery
Safety and effectiveness of 0.9% Sodium Chloride Irrigation USP during labor and delivery have not been established. Caution should be exercised, and the fluid balance, glucose and electrolyte concentrations, and acid-base balance, of both mother and fetus should be evaluated periodically or whenever warranted by the condition of the patient or fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when 0.9% Sodium Chloride Irrigation USP is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of 0.9% Sodium Chloride Irrigation USP in pediatric patients have not been established. Its limited use in pediatric patients has been inadequate to fully define proper dosage and limitations for use.
Geriatric Use
Clinical studies of 0.9% Sodium Chloride Irrigation USP did not include a sufficient number of patients age 65 years and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Frequent laboratory determinations and clinical evaluations are recommended to monitor changes in blood glucose, electrolyte concentrations, and renal function.
Adverse Reactions/Side Effects
Possible adverse effects arising from the irrigation of body cavities, tissues, or indwelling catheters and tubes can be minimized when proper procedures are followed. Displaced catheters or drainage tubes can lead to irrigation or infiltration of unintended structures or cavities. Excessive volume or pressure during irrigation of closed cavities may cause undue distension or disruption of tissues. Accidental contamination from careless technique may transmit infection.
If an adverse reaction does occur, discontinue administration of the irrigant, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
Overdosage
In the event of overhydration or solute overload, reevaluate the patient’s condition, and institute appropriate corrective treatment. Intravascular volume overload may respond to hemodialysis. See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
Sodium Chloride Irrigation Dosage and Administration
As required for irrigation.
When used as a diluent, or vehicle for other drugs, the drug manufacturer’s recommendations should be followed.
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Solutions should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permits.
How is Sodium Chloride Irrigation supplied
0.9% Sodium Chloride Irrigation USP is supplied sterile and nonpyrogenic in PIC™ (Plastic Irrigation Container). The 1000 mL and 500 mL containers are packaged 16 per case.
| NDC | Cat. No. | Size |
|---|---|---|
| 0.9% Sodium Chloride Irrigation USP | ||
| 0264-2201-00 | R5200-01 | 1000 mL |
| 0264-2201-10 | R5201-01 | 500 mL |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature.] However, brief exposure up to 40°C does not adversely affect the product.
Do not warm above 150°F (66°C).
Directions for Use of PIC™ (Plastic Irrigation Container)
Not for injection.
Aseptic technique is required.
- Caution – Before use, perform the following checks:
(a) Read the label. Ensure solution is the one ordered and is within the expiration date.
(b) Invert container and inspect the solution in good light for cloudiness, haze, or particulate matter; check the
container for leakage or damage. Any container which is suspect should not be used.
Use only if solution is clear and container and seal are intact.
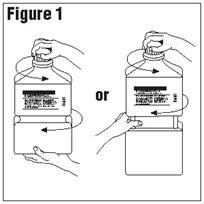
Single-dose container. Discard unused portion. - Outer Closure Removal – Grasp the container with one hand and turn the breakaway ring counterclockwise with the other hand until slight resistance is felt. Then, twisting the container in the opposite direction, turn the breakaway ring sharply until the entire outer cap is loose and can be lifted off. (Figure 1)
- Connect the administration set through the sterile set port according to set instructions (Figure 2) or remove screw cap and pour.
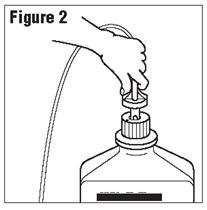
- Do not warm above 150°F (66°C) to assure minimal bottle distortion. Keep bottles upright.
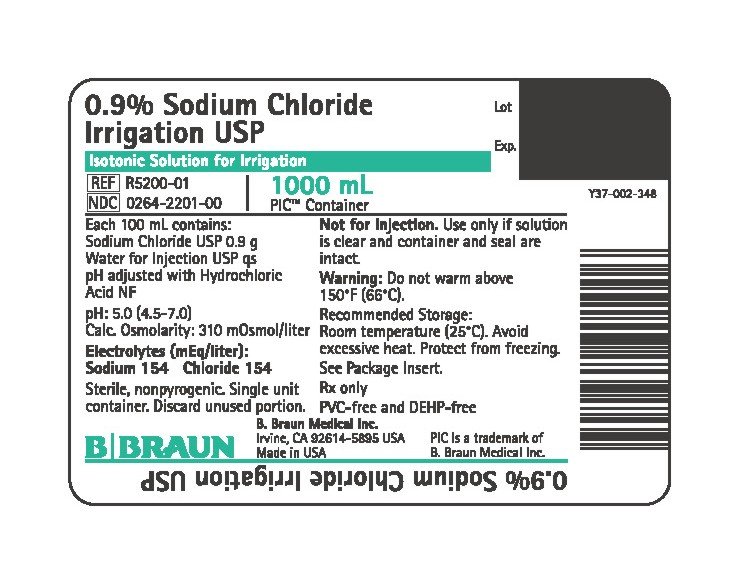
PRINCIPAL DISPLAY PANEL - 1000 mL Container
0.9% Sodium Chloride Irrigation USP
Isotonic Solution for Irrigation
REF R5200-01
NDC 0264-2201-00
1000 mL
PIC™ Container
Rx only
Lot
Exp.
Each 100 mL contains:
Sodium Chloride USP 0.9 g
Water for Injection USP qs
pH adjusted with Hydrochloric
Acid NF
pH: 5.0 (4.5-7.0)
Calc. Osmolarity: 310 mOsmol/liter
Electrolytes (mEq/liter):
Sodium 154 Chloride 154
Sterile, nonpyrogenic. Single-dose container. Discard unused portion.
Not for Injection. Use only if solution is clear and container and seal are intact.
Warning: Do not warm above 150°F (66°C).
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.] Avoid excessive heat. Protect from freezing.
Dosage: See Prescribing Information.
Not made with natural rubber latex, PVC or DEHP.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Y37-002-590 LD-770-1
0.9% Sodium Chloride Irrigation USP
PRINCIPAL DISPLAY PANEL - 500 mL Container
0.9% Sodium Chloride Irrigation USP
Isotonic Solution for Irrigation
REF R5201-01
NDC 0264-2201-10
500 mL
PIC™ Container
Rx only
Lot
Exp.
Each 100 mL contains:
Sodium Chloride USP 0.9 g
Water for Injection USP qs
pH adjusted with Hydrochloric
Acid NF
pH: 5.0 (4.5-7.0)
Calc. Osmolarity: 310 mOsmol/liter
Electrolytes (mEq/liter):
Sodium 154 Chloride 154
Sterile, nonpyrogenic. Single-dose container. Discard unused portion.
Not for Injection. Use only if solution is clear and container and seal are intact.
Warning: Do not warm above 150°F (66°C).
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.] Avoid excessive heat. Protect from freezing.
Dosage: See Prescribing Information.
Not made with natural rubber latex, PVC or DEHP.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Y37-002-591 LD-771-1
0.9% Sodium Chloride Irrigation USP

| SODIUM CHLORIDE
sodium chloride irrigant |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |
