Priscoline: Package Insert / Prescribing Info
Package insert / product label
Generic name: tolazoline hydrochloride
Dosage form: Injection
Drug class: Miscellaneous cardiovascular agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
C98-24
665480
Priscoline® hydrochloride
tolazoline hydrochloride injection, USP
Ampuls
Rx only
Prescribing Information
Priscoline Description
Priscoline hydrochloride, tolazoline hydrochloride injection, USP, is a peripheral vasodilator available in ampuls for intravenous administration. Each milliliter of sterile, aqueous solution contains tolazoline hydrochloride USP, 25 mg; tartaric acid ACS, 6.5 mg; and hydrous sodium citrate USP, 6.5 mg. Tolazoline hydrochloride is 4,5-dihydro-2-(phenylmethyl)-
1H-imidazole monohydrochloride, and its structural formula is
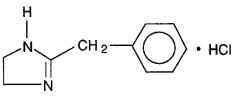
Tolazoline hydrochloride USP is a white to off-white crystalline powder. Its solutions are slightly acid to litmus. It is freely soluble in water and in alcohol. Its molecular weight is 196.68.
Priscoline - Clinical Pharmacology
Priscoline is a direct peripheral vasodilator with moderate competitive alpha-adrenergic blocking activity. It decreases peripheral resistance and increases venous capacitance. It has the following additional actions: (1) sympathomimetic, including cardiac stimulation; (2) parasympathomimetic, including gastrointestinal tract stimulation that is blocked by atropine; and (3) histamine-like, including stimulation of gastric secretion and peripheral vasodilatation. Priscoline given intravenously produces vasodilatation, primarily due to a direct effect on vascular smooth muscle, and cardiac stimulation; the blood pressure response depends on the relative contributions of the two effects. Priscoline usually reduces pulmonary arterial pressure and vascular resistance.
In neonates the half-life of Priscoline ranges from 3 to 10 hours.
Indications and Usage for Priscoline
Priscoline is indicated for the treatment of persistent pulmonary hypertension of the newborn (“persistent fetal circulation”) when systemic arterial oxygenation cannot be satisfactorily maintained by usual supportive care (supplemental oxygen and/or mechanical ventilation).
Priscoline should be used in a highly supervised setting, where vital signs, oxygenation, acid-base status, fluid, and electrolytes can be monitored and maintained.
Warnings
Priscoline stimulates gastric secretion and may activate stress ulcers. Through this mechanism, it can produce significant hypochloremic alkalosis. Pretreatment of infants with antacids may prevent gastrointestinal bleeding.
Patients should be observed closely for signs of systemic hypotension, and supportive therapy should be instituted if needed.
In patients with mitral stenosis, parenterally administered Priscoline may produce a rise or fall in pulmonary artery pressure and total pulmonary resistance; therefore, it must be used with caution in patients with known or suspected mitral stenosis.
Precautions
General
The effects of Priscoline on pulmonary vessels may be pH dependent. Acidosis may decrease the effect of Priscoline.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals have not been performed with Priscoline.
Pregnancy Category C
Animal reproduction studies have not been conducted with Priscoline. It is also not known whether Priscoline can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Priscoline should be given to a pregnant woman only if clearly needed.
Adverse Reactions/Side Effects
The following adverse reactions have been observed, but there are insufficient data to support an estimate of their frequency:
Cardiovascular: Hypotension, tachycardia, cardiac arrhythmias, hypertension, pulmonary hemorrhage.
Digestive and Hepatic: Gastrointestinal hemorrhage, nausea, vomiting, diarrhea, hepatitis.
Skin: Flushing, increased pilomotor activity with tingling or chilliness, rash.
Hematologic: Thrombocytopenia, leukopenia.
Renal: Edema, oliguria, hematuria.
Related/similar drugs
Overdosage
Priscoline Dosage and Administration
An initial dose of 1 to 2 mg/kg, via scalp vein, followed by an infusion of 1 to 2 mg/kg per hour have usually resulted in significant increases in arterial oxygen. There is very little experience with infusions lasting beyond 36 to 48 hours. Response, if it occurs, can be expected within 30 minutes after the initial dose.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
How is Priscoline supplied
Ampuls 4 mL— each milliliter contains 25 mg of tolazoline hydrochloride.
Carton of 4 ampuls……………………………………………………….NDC 0083-6733-04
Store between 15ºC and 30ºC (59ºF-86ºF).
Protect from light.
665480 C98-24 (Rev. 9/98)
Distributed by
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
©1998 Novartis
| PRISCOLINE HYDROCHLORIDE
tolazoline hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation |
