Oticin Ear Drops Prescribing Information
Package insert / product label
Generic name: chloroxylenol and pramoxine hydrochloride
Dosage form: ear drops
Drug class: Otic anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Oticin Ear Drops Description
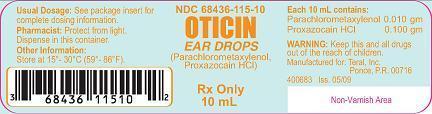
Each 10 mL for otic administration contains:
Parachlorometaxylenol …..….. 0.010 gm
Proxazocain HCl …………..…. 0.100 gm
Parachlorometaxylenol (4-chloro-3,5-xylenol) is a wide spectrum antimicrobial agent.
Proxazocain (p-butoxyphenyl γ-morpholinopropyl ether), used as the hydrochloride salt, is a topical anesthetic.
Oticin Ear Drops - Clinical Pharmacology
Parachlorometaxylenol in low concentrations is a germicide that may be used to treat bacterial and fungal infections. It is a halogenated phenol, non-toxic, non-corrosive, non-staining with a high phenol coefficient. It may be applied directly to a wound and shows no chemical reactivity toward blood.
Proxazocain HCl is a topical anesthetic which is chemically unrelated to procaine and the other "caines". It provides temporary relief from itching and pain by stabilizing the neuronal membranes of nerve endings with which it comes into contact.
Indications and Usage for Oticin Ear Drops
For the treatment of superficial infections of the external ear caused by microbes and to control the accompanying itching.
Related/similar drugs
Ciprodex, ceftazidime, Cortisporin Otic, Cortisporin-TC, Pediotic
Contraindications
This medication is contraindicated in patients sensitive to any of the components of this preparation. This medication should not be applied in the external auditory canal where there is a perforated eardrum or when this medication can reach the middle ear.
Warnings
This product is not intended for ophthalmic or oral use. If irritation or sensitization occurs, promptly discontinue use of this preparation and institute other measures.
Precautions
General
Treatment should not be continued for longer than ten days and the source of infection and/or inflammation or sensitization evaluated to determine whether therapy should be changed.
Information for Patients: Patients using this medication should be advised of the following:
• This medication is to be used as directed by a physician.
• Do not use this medication for any disorder other than for which it was prescribed. Check with your physician before using this medication for future ear problems.
• Report any signs of local adverse reactions to your physician.
• Keep this and all medications out of the reach of children. In case of accidental overdose or ingestion, seek professional assistance or contact a poison control center immediately.
• Store at controlled room temperature between 15(- 30(C (59(- 86(F).
Oticin Ear Drops Dosage and Administration
The external ear that is the auricle and the auditory canal should be thoroughly cleansed and dried. Four (4) to five (5) drops of Oticin Ear Drops should be instilled into the affected ear 3 to 4 times daily. For infants and small children, 3 drops are suggested because of the smaller capacity of the ear canal. The patient should lie with the affected ear upward to instill the drops and this position maintained for at least 5 minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary for the opposite affected ear. If preferred, a gauze ear plug or wick may be inserted into the ear canal prior to applying Oticin Ear Drops. When using a wick, a few drops of sterile water should be applied directly to the wick following insertion to facilitate hydration. It should then be saturated with Oticin Ear Drops. The patient should be instructed to add a few drops of Oticin Ear Drops to the wick every four hours, and to remove the wick after 24 hours. Five (5) drops of Oticin Ear Drops should continue to be instilled 3 or 4 times daily thereafter. When using a gauze earplug, the external ear canal is first treated with drops of Oticin Ear Drops. Next, the gauze is inserted into the ear canal to contain medication. The gauze ear plug or the expanded wick are easily removed by fingers, forceps, or tweezers. This product may continue to be used in the unaffected ear 3 times daily as a preventative.
How is Oticin Ear Drops supplied
Oticin Ear Drops are supplied as a clear liquid in plastic dropper vials of 10 mL, NDC 68436-115-10.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
| OTICIN
chloroxylenol / pramoxine hcl solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Teral, Inc. (186958810) |
| Registrant - Teral, Inc. (186958810) |