Lastacaft: Package Insert / Prescribing Info
Package insert / product label
Generic name: alcaftadine
Dosage form: ophthalmic solution
Drug class: Ophthalmic antihistamines and decongestants
Medically reviewed by Drugs.com. Last updated on Jan 16, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
These highlights do not include all the information needed to use LASTACAFT® safely and effectively. See full prescribing information for LASTACAFT®.
LASTACAFT® (alcaftadine ophthalmic solution),
for topical ophthalmic use
Initial U.S. Approval: 2010
Indications and Usage for Lastacaft
LASTACAFT® is an H1 histamine receptor antagonist indicated for the prevention of itching associated with allergic conjunctivitis. (1)
Lastacaft Dosage and Administration
Instill one drop in each eye once daily. (2)
Dosage Forms and Strengths
Ophthalmic solution containing alcaftadine 0.25% (2.5 mg/mL) (3)
Contraindications
Hypersensitivity (4)
Warnings and Precautions
• Potential for Eye Injury and Contamination: To minimize the risk of eye injury and contamination, do not touch dropper tip to eyelids and surrounding areas, or any other surface. Keep bottle tightly closed when not in use. (5.1)
• Contact Lens Wear: LASTACAFT® should not be used to treat contact lens-related irritation. Remove contact lenses prior to instillation of LASTACAFT®. (5.2)
Adverse Reactions/Side Effects
The most common ocular adverse reactions, occurring in less than 4% of eyes treated with LASTACAFT®, were eye irritation, burning and/or stinging on instillation, eye redness, and eye pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2020
Full Prescribing Information
1. Indications and Usage for Lastacaft
LASTACAFT® is an H1 histamine receptor antagonist indicated for the prevention of itching associated with allergic conjunctivitis.
2. Lastacaft Dosage and Administration
Instill one drop in each eye once daily. If more than 1 topical ophthalmic medicinal product is being used, each one should be administered at least 5 minutes apart.
4. Contraindications
LASTACAFT® is contraindicated in patients with hypersensitivity to any component in the product.
5. Warnings and Precautions
5.1 Potential for Eye Injury and Contamination
To minimize eye injury and contamination of the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
5.2 Contact Lens Use
Patients should be advised not to wear a contact lens if their eye is red.
LASTACAFT® should not be used to treat contact lens-related irritation.
LASTACAFT® should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of LASTACAFT®. The preservative in LASTACAFT®, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of LASTACAFT®.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most frequent ocular adverse reactions, occurring in less than 4% of eyes treated with LASTACAFT®, were eye irritation, burning and/or stinging upon instillation, eye redness and eye pruritus.
The most frequent non-ocular adverse reactions, occurring in less than 3% of subjects with eyes treated with LASTACAFT®, were nasopharyngitis and headache. Some of these events were similar to the underlying disease being studied.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of LASTACAFT®. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These reactions include eye discharge, eye swelling, erythema of eyelid, eyelid edema, lacrimation increased, vision blurred, hypersensitivity reactions including swelling of the face or allergic dermatitis, and somnolence.
Related/similar drugs
Ozempic
Learn about Ozempic (semaglutide) for type 2 diabetes treatment, weight management, cardiovascular ...
Cromolyn ophthalmic
Cromolyn ophthalmic is used for allergies, conjunctivitis, allergic, keratitis, keratoconjunctivitis
Ketotifen ophthalmic
Ketotifen ophthalmic is used for allergies, conjunctivitis, allergic
Doxylamine
Doxylamine is used for allergic rhinitis, allergies, conjunctivitis, allergic, insomnia, nasal ...
Deltasone
Deltasone is used for acute lymphocytic leukemia, adrenocortical insufficiency, allergic reactions ...
Prednisolone ophthalmic
Prednisolone ophthalmic is used for allergies, conjunctivitis, allergic, iritis, keratitis ...
Azelastine ophthalmic
Azelastine ophthalmic is used for conjunctivitis, allergic
Dexamethasone ophthalmic
Dexamethasone ophthalmic is used for conjunctivitis, conjunctivitis, allergic, cyclitis, diabetic ...
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with LASTACAFT® in pregnant women to inform a drug associated risk. There are limited data with the use of alcaftadine eye drops in pregnant women.
In embryofetal studies in rats and rabbits, oral administration of alcaftadine during the period of organogenesis did not produce maternal or embryofetal toxicity at clinically relevant doses.
Advise pregnant women of a potential risk to the fetus and mother. LASTACAFT® should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus and mother. The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data
Animal Data
In rats, oral administration of 5, 20 or 40 mg/kg/day alcaftadine during the period of organogenesis (gestational days 6 – 16) caused maternal lethality at doses of 40 mg/kg. The no observed adverse effect level (NOAEL) for maternal toxicity was 20 mg/kg/day (an exposure 230-times higher than that at the maximum recommended human ophthalmic dose [MRHOD], based on AUC). There were no adverse embryofetal effects up to a dose of 20 mg/kg.
In rabbits, oral administration of 10, 40 or 80 mg/kg/day alcaftadine during the period of organogenesis (gestational days 6 – 18) caused no maternal toxicity or adverse embryofetal effects up to a dose of 80 mg/kg/day (an exposure 8819-times higher than that at the MRHOD, based on AUC).
Daily oral doses of 20 and 30 mg/kg/day alcaftadine administered to rats from Day 6 of pregnancy until Day 20 postpartum produced lower pup weights in offspring. No adverse effects in dams or offspring were observed at doses up to 5 mg/kg/day (a dose 286 times higher than the MRHOD, on a mg/m2 basis).
8.2 Lactation
Risk Summary
There is no information regarding the presence of LASTACAFT® in human milk, the effects on the breastfed infants, or the effects on milk production to inform risk of LASTACAFT® to an infant during lactation. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for LASTACAFT®, and any potential adverse effects on the breastfed infant from LASTACAFT®.
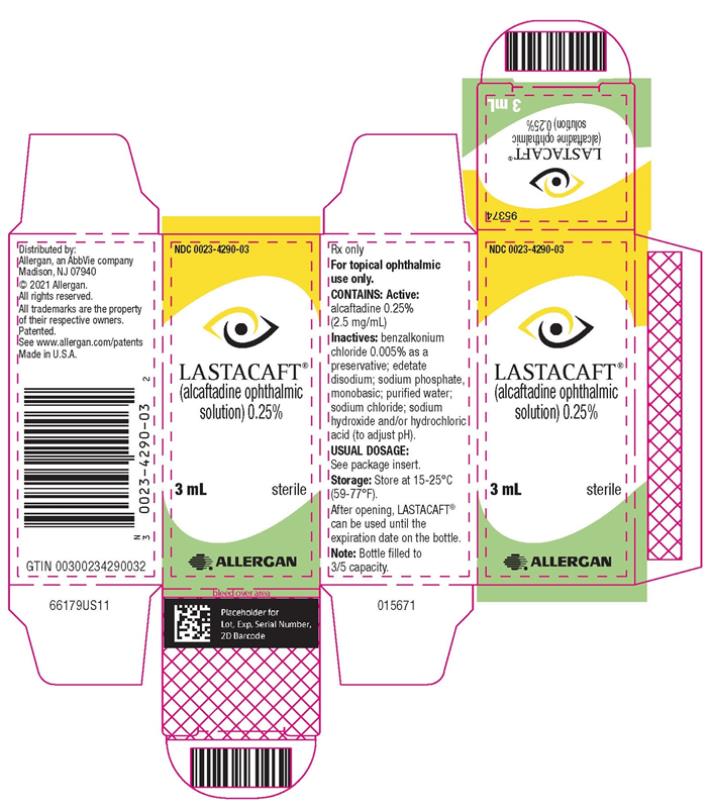
11. Lastacaft Description
LASTACAFT® (alcaftadine ophthalmic solution) is an H1 receptor antagonist, in a sterile ophthalmic solution for topical ophthalmic use.
Alcaftadine is a white to yellow powder with an empirical formula of C19H21N3O and a molecular weight of 307.39.
Contains:
Active: alcaftadine 0.25% (2.5 mg/mL)
Inactives: benzalkonium chloride 0.005% as a preservative; edetate disodium; sodium phosphate, monobasic; purified water; sodium chloride; sodium hydroxide and/or hydrochloric acid (to adjust pH)
Chemical Name: 6,11-dihydro-11-(1-methyl-4-piperidinylidene)-5H-imidazo[2,1-b] [3] benzazepine-3-carboxaldehyde
Structural Formula:
![The chemical structure for Chemical Name: 6,11-dihydro-11-(1-methyl-4-piperidinylidene)-5H-imidazo[2,1-b] [3] benzazepine-3-carboxaldehyde](https://www.drugs.com/pro/images/17d37e8d-7825-424b-b6ca-0a85d0bf4694/lastacaft-01.jpg)
The drug product has a pH of approximately 7 and an osmolality of approximately 290 mOsm/kg.
12. Lastacaft - Clinical Pharmacology
12.1 Mechanism of Action
Alcaftadine is an H1 histamine receptor antagonist and inhibitor of the release of histamine from mast cells. Decreased chemotaxis and inhibition of eosinophil activation has also been demonstrated.
12.3 Pharmacokinetics
Absorption
Following bilateral topical ocular administration of alcaftadine ophthalmic solution, 0.25%, the mean plasma Cmax of alcaftadine was approximately 60 pg/mL and the median Tmax occurred at 15 minutes. Plasma concentrations of alcaftadine were below the lower limit of quantification (10 pg/mL) by 3 hours after dosing. The mean Cmax of the active carboxylic acid metabolite was approximately 3 ng/mL and occurred at 1 hour after dosing. Plasma concentrations of the carboxylic acid metabolite were below the lower limit of quantification (100 pg/mL) by 12 hours after dosing. There was no indication of systemic accumulation or changes in plasma exposure of alcaftadine or the active metabolite following daily topical ocular administration.
Distribution
The protein binding of alcaftadine and the active metabolite are 39.2% and 62.7%, respectively.
Elimination
Metabolism
The metabolism of alcaftadine is mediated by non-CYP450 cytosolic enzymes to the active carboxylic acid metabolite. In vitro studies showed that neither alcaftadine nor the carboxylic acid metabolite substantially inhibited reactions catalyzed by major CYP450 enzymes.
Excretion
The elimination half-life of the carboxylic acid metabolite is approximately 2 hours following topical ocular administration. Based on data following oral administration of alcaftadine, the carboxylic acid metabolite is primarily eliminated unchanged in the urine.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of alcaftadine has not been evaluated in long-term animal studies.
Mutagenesis
Alcaftadine was not mutagenic or genotoxic in the Ames test, the mouse lymphoma assay or the mouse micronucleus assay.
Impairment of Fertility
Alcaftadine was found to have no effect on fertility of male and female rats at oral doses up to 20 mg/kg/day (an exposure 230-times higher than that at the MRHOD, based on AUC).
14. Clinical Studies
Clinical efficacy was evaluated in conjunctival allergen challenge (CAC) studies.
LASTACAFT® was more effective than its vehicle in preventing ocular itching in patients with allergic conjunctivitis induced by an ocular allergen challenge, both at 3 minutes post-dosing and at 16 hours post-dosing of LASTACAFT®.
The safety of LASTACAFT® was evaluated in a randomized clinical study of 909 subjects over a period of 6 weeks.
16. How is Lastacaft supplied
LASTACAFT® (alcaftadine ophthalmic solution) 0.25% is supplied in an opaque, white low-density polyethylene bottle with a white polystyrene cap.
3 mL fill in 5 mL bottle NDC 0023-4290-03
Storage: Store at 15°C to 25°C (59°F to 77°F).
After opening, LASTACAFT® can be used until the expiration date on the bottle.
17. Patient Counseling Information
Potential for Eye Injury and Sterility of Dropper Tip
To minimize eye injury and contamination of the dropper tip and solution, advise patients to not touch the eyelids or surrounding areas with the dropper tip, as this may contaminate the contents.
Concomitant Use with other Ophthalmic Products
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
Contact Lens Use
Advise patients not to wear a contact lens if their eye is red. LASTACAFT® should not be used to treat contact lens-related irritation. Advise patients remove contact lenses prior to instillation of LASTACAFT®. The preservative in LASTACAFT®, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of LASTACAFT®.
Distributed by: Allergan, an AbbVie company
Madison, NJ 07940
© 2020 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Patented. See www.allergan.com/patents
v1.0USPI4290
| LASTACAFT
alcaftadine solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |
More about Lastacaft (alcaftadine ophthalmic)
- Compare alternatives
- Pricing & coupons
- Reviews (30)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: ophthalmic antihistamines and decongestants
- En español