Hydroxyurea Prescribing Information
Package insert / product label
Dosage form: capsule
Drug class: Antimetabolites
Medically reviewed by Drugs.com. Last updated on Apr 16, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
HYDROXYUREA capsules, for oral use
Initial U.S. Approval: 1967
Recent Major Changes
Indications and Usage for Hydroxyurea
Hydroxyurea Dosage and Administration
Dosage Forms and Strengths
- Capsules: 500 mg (3)
Contraindications
- In patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation. (4)
Warnings and Precautions
- Myelosuppression: Do not give if bone marrow function is markedly depressed. Monitor blood counts at baseline and throughout treatment. Interrupt treatment and reduce dose as necessary. (5.1)
- Hemolytic anemia: Monitor blood counts throughout treatment. If hemolysis persists, discontinue hydroxyurea. (5.2)
- Malignancies: Advise protection from sun exposure and monitor for secondary malignancies. (5.3)
- Embryo-Fetal toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception. (5.4, 8.1, 8.3)
- Vasculitic toxicities: Discontinue hydroxyurea and initiate treatment if this occurs. (5.5)
- Live Vaccinations: Avoid live vaccine use in a patient taking hydroxyurea capsules. (5.6)
- Risks with concomitant use of antiretroviral drugs: Pancreatitis, hepatotoxicity, and neuropathy have occurred. Monitor for signs and symptoms in patients with HIV infection using antiretroviral drugs; discontinue hydroxyurea capsules and implement treatment. (5.7)
- Radiation recall: Monitor for skin erythema in patients who previously received radiation and manage symptomatically. (5.8)
Adverse Reactions/Side Effects
Most common adverse reactions (≥30%) are hematological, gastrointestinal symptoms, and anorexia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact QILU PHARMA, INC. at 484-838-0633 / 484-875-3013 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2024
Full Prescribing Information
1. Indications and Usage for Hydroxyurea
Hydroxyurea capsules are indicated for the treatment of:
- •
- Resistant chronic myeloid leukemia.
- •
- Locally advanced squamous cell carcinomas of the head and neck (excluding the lip) in combination with chemoradiation.
2. Hydroxyurea Dosage and Administration
2.1 Dosing Information
Hydroxyurea is used alone or in conjunction with other antitumor agents or radiation therapy to treat neoplastic diseases. Individualize treatment based on tumor type, disease state, response to treatment, patient risk factors, and current clinical practice standards.
Base all dosage on the patient’s actual or ideal weight, whichever is less.
Hydroxyurea is a cytotoxic drug. Follow applicable special handling and disposal procedures [see References (15)].
Swallow hydroxyurea capsules whole. Do NOT open, break, or chew capsules because hydroxyurea is a cytotoxic drug.
Prophylactic administration of folic acid is recommended [see Warnings and Precautions (5.8)].
Monitor blood counts at least once a week during hydroxyurea therapy. Severe anemia must be corrected before initiating therapy with hydroxyurea.
2.2 Dose Modifications for Toxicity
Monitor for the following and reduce the dose or discontinue hydroxyurea accordingly:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Cutaneous vasculitis [see Warnings and Precautions (5.5)]
Consider dose modifications for other toxicities.
2.3 Dose Modifications for Renal Impairment
Reduce the dose of hydroxyurea capsules by 50% in patients with measured creatinine clearance of less than 60 mL/min or with end-stage renal disease (ESRD) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
|
Creatinine Clearance (mL/min) |
Recommended Hydroxyurea Capsules Initial Dose (mg/kg once daily) |
|
≥60 |
15 |
|
<60 or ESRD* |
7.5 |
* On dialysis days, administer hydroxyurea capsules to patients following hemodialysis.
Close monitoring of hematologic parameters is advised in these patients.
3. Dosage Forms and Strengths
Capsules: 500 mg opaque pink body and opaque powder blue cap, imprinted with “83” on the body in black ink.
4. Contraindications
Hydroxyurea capsules are contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of the formulation.
5. Warnings and Precautions
5.1 Myelosuppression
Hydroxyurea causes severe myelosuppression. Treatment with hydroxyurea should not be initiated if bone marrow function is markedly depressed. Bone marrow suppression may occur, and leukopenia is generally its first and most common manifestation. Thrombocytopenia and anemia occur less often and are seldom seen without a preceding leukopenia. Bone marrow depression is more likely in patients who have previously received radiotherapy or cytotoxic cancer chemotherapeutic agents; use hydroxyurea cautiously in such patients.
Evaluate hematologic status prior to and during treatment with hydroxyurea capsules. Provide supportive care and modify dose or discontinue hydroxyurea as needed. Recovery from myelosuppression is usually rapid when therapy is interrupted.
5.2 Hemolytic Anemia
Cases of hemolytic anemia in patients treated with hydroxyurea for myeloproliferative diseases have been reported [see Adverse Reactions (6.1)]. Patients who develop acute jaundice or hematuria in the presence of persistent or worsening of anemia should have laboratory tests evaluated for hemolysis (e.g., measurement of serum lactate dehydrogenase, haptoglobin, reticulocyte, unconjugated bilirubin levels, urinalysis, and direct and indirect antiglobulin [Coombs] tests). In the setting of confirmed diagnosis of hemolytic anemia and in the absence of other causes, discontinue hydroxyurea.
5.3 Malignancies
Hydroxyurea is a human carcinogen. In patients receiving long-term hydroxyurea for myeloproliferative disorders, secondary leukemia has been reported. Skin cancer has also been reported in patients receiving long-term hydroxyurea. Advise protection from sun exposure and monitor for the development of secondary malignancies.
5.4 Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, hydroxyurea can cause fetal harm when administered to a pregnant woman. Hydroxyurea was embryotoxic and teratogenic in rats and rabbits at doses 0.8 times and 0.3 times, respectively, the maximum recommended human daily dose on a mg/m2 basis. Advise pregnant women of the potential risk to a fetus [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during and after treatment with hydroxyurea capsules for at least 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with hydroxyurea capsules for at least 1 year after therapy [see Use in Specific Populations (8.1, 8.3)].
5.5 Vasculitic Toxicities
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. These vasculitic toxicities were reported most often in patients with a history of, or currently receiving, interferon therapy. If cutaneous vasculitic ulcers occur, institute treatment and discontinue hydroxyurea capsules.
5.6 Live Vaccinations
Avoid use of live vaccine in patients taking hydroxyurea capsules. Concomitant use of hydroxyurea capsules with a live virus vaccine may potentiate the replication of the virus and/or may increase the adverse reaction of the vaccine because normal defense mechanisms may be suppressed by hydroxyurea capsules. Vaccination with live vaccines in a patient receiving hydroxyurea capsules may result in severe infection. Patient’s antibody response to vaccines may be decreased. Consider consultation with a specialist.
5.7 Risks with Concomitant Use of Antiretroviral Drugs
Pancreatitis, hepatotoxicity, and peripheral neuropathy have occurred when hydroxyurea was administered concomitantly with antiretroviral drugs, including didanosine and stavudine [see Drug Interactions (7.1)].
5.8 Radiation Recall
Patients who have received irradiation therapy in the past may have an exacerbation of post-irradiation erythema. Monitor for skin erythema in patients who previously received radiation and manage symptomatically.
5.9 Macrocytosis
Hydroxyurea capsules may cause macrocytosis, which is self-limiting, and is often seen early in the course of treatment. The morphologic change resembles pernicious anemia, but is not related to vitamin B12 or folic acid deficiency. This may mask the diagnosis of pernicious anemia. Prophylactic administration of folic acid is recommended.
5.10 Pulmonary Toxicity
Interstitial lung disease including pulmonary fibrosis, lung infiltration, pneumonitis, and alveolitis/allergic alveolitis (including fatal cases) have been reported in patients treated for myeloproliferative neoplasm. Monitor patients developing pyrexia, cough, dyspnea, or other respiratory symptoms frequently, investigate and treat promptly. Discontinue hydroxyurea and manage with corticosteroids [see Adverse Reactions (6.1)].
5.11 Laboratory Test Interference
Interference with Uric Acid, Urea, or Lactic Acid Assays is possible, rendering falsely elevated results of these in patients treated with hydroxyurea [see Drug Interactions (7.2)].
Hydroxyurea may falsely elevate sensor glucose results from certain continuous glucose monitoring (CGM) systems and may lead to hypoglycemia if sensor glucose results are relied upon to dose insulin.
If a patient using a CGM is to be prescribed hydroxyurea, consult with the CGM prescriber about alternative glucose monitoring methods [see Drug Interactions (7.2)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described in detail in other labeling sections:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Hemolytic anemia [see Warnings and Precautions (5.2)]
- Malignancies [see Warnings and Precautions (5.3)]
- Vasculitic toxicities [see Warnings and Precautions (5.5)]
- Risks with concomitant use of antiretroviral drugs [see Warnings and Precautions (5.7)]
- Radiation recall [see Warnings and Precautions (5.8)]
-
Macrocytosis [see Warnings and Precautions (5.9)]
-
Pulmonary Toxicity [see Warnings and Precautions (5.10)]
6.1 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of hydroxyurea capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
- Reproductive System and Breast disorders: azoospermia, and oligospermia
- Gastrointestinal disorders: stomatitis, nausea, vomiting, diarrhea, and constipation
- Metabolism and Nutrition disorders: anorexia, tumor lysis syndrome
- Skin and subcutaneous tissue disorders: maculopapular rash, skin ulceration, cutaneous lupus erythematosus, dermatomyositis-like skin changes, peripheral and facial erythema, hyperpigmentation, nail hyperpigmentation, atrophy of skin and nails, scaling, violet papules, and alopecia
- Renal and urinary disorders: dysuria, elevations in serum uric acid, blood urea nitrogen (BUN), and creatinine levels
- Nervous system disorders: headache, dizziness, drowsiness, disorientation, hallucinations, and convulsions
- General Disorders: fever, chills, malaise, edema, and asthenia
- Hepatobiliary disorders: elevation of hepatic enzymes, cholestasis, and hepatitis
- Respiratory disorders: diffuse pulmonary infiltrates, dyspnea, and pulmonary fibrosis, interstitial lung disease, pneumonitis, alveolitis, allergic alveolitis and cough
- Immune disorders: systemic lupus erythematosus
- Hypersensitivity: Drug-induced fever (pyrexia) (>39°C, >102°F) requiring hospitalization has been reported concurrently with gastrointestinal, pulmonary, musculoskeletal, hepatobiliary, dermatological or cardiovascular manifestations. Onset typically occurred within 6 weeks of initiation and resolved upon discontinuation of hydroxyurea. Upon re-administration fever re-occurred typically within 24 hours.
- Blood and lymphatic system disorders: hemolytic anemia
Adverse reactions observed with combined hydroxyurea and irradiation therapy are similar to those reported with the use of hydroxyurea or radiation treatment alone. These effects primarily include bone marrow depression (anemia and leukopenia), gastric irritation, and mucositis. Almost all patients receiving an adequate course of combined hydroxyurea and irradiation therapy will demonstrate concurrent leukopenia. Platelet depression (<100,000 cells/mm3) has occurred in the presence of marked leukopenia. Hydroxyurea capsules may potentiate some adverse reactions usually seen with irradiation alone, such as gastric distress and mucositis.
7. Drug Interactions
7.1 Increased Toxicity with Concomitant Use of Antiretroviral Drugs
Pancreatitis
In patients with HIV infection during therapy with hydroxyurea and didanosine, with or without stavudine, fatal and nonfatal pancreatitis have occurred. Hydroxyurea is not indicated for the treatment of HIV infection; however, if patients with HIV infection are treated with hydroxyurea, and in particular, in combination with didanosine and/or stavudine, close monitoring for signs and symptoms of pancreatitis is recommended. Permanently discontinue therapy with hydroxyurea in patients who develop signs and symptoms of pancreatitis.
Hepatotoxicity
Hepatotoxicity and hepatic failure resulting in death have been reported during postmarketing surveillance in patients with HIV infection treated with hydroxyurea and other antiretroviral drugs. Fatal hepatic events were reported most often in patients treated with the combination of hydroxyurea, didanosine, and stavudine. Avoid this combination.
7.2 Laboratory Test Interference
Interference with Uric Acid, Urea, or Lactic Acid Assays
Studies have shown that there is an analytical interference of hydroxyurea with the enzymes (urease, uricase, and lactate dehydrogenase) used in the determination of urea, uric acid, and lactic acid, rendering falsely elevated results of these in patients treated with hydroxyurea.
Interference with Continuous Glucose Monitoring Systems
Hydroxyurea may falsely elevate sensor glucose results from certain continuous glucose monitoring (CGM) systems and may lead to hypoglycemia if sensor glucose results are relied upon to dose insulin.
If a patient using a CGM is to be prescribed hydroxyurea, consult with the CGM prescriber about alternative glucose monitoring methods.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Hydroxyurea capsules can cause fetal harm based on findings from animal studies and the drug’s mechanism of action [see Clinical Pharmacology (12.1)]. There are no data with hydroxyurea capsules use in pregnant women to inform a drug-associated risk. In animal reproduction studies, administration of hydroxyurea to pregnant rats and rabbits during organogenesis produced embryotoxic and teratogenic effects at doses 0.8 times and 0.3 times, respectively, the maximum recommended human daily dose on a mg/m2 basis (see Data). Advise women of the potential risk to a fetus and to avoid becoming pregnant while being treated with hydroxyurea capsules.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and 15%–20%, respectively.
Data
Animal Data
Hydroxyurea has been demonstrated to be a potent teratogen in a wide variety of animal models, including mice, hamsters, cats, miniature swine, dogs, and monkeys at doses within 1-fold of the human dose given on a mg/m2 basis. Hydroxyurea is embryotoxic and causes fetal malformations (partially ossified cranial bones, absence of eye sockets, hydrocephaly, bipartite sternebrae, missing lumbar vertebrae) at 180 mg/kg/day (about 0.8 times the maximum recommended human daily dose on a mg/m2 basis) in rats and at 30 mg/kg/day (about 0.3 times the maximum recommended human daily dose on a mg/m2 basis) in rabbits. Embryotoxicity was characterized by decreased fetal viability, reduced live litter sizes, and developmental delays. Hydroxyurea crosses the placenta. Single doses of ≥375 mg/kg (about 1.7 times the maximum recommended human daily dose on a mg/m2 basis) to rats caused growth retardation and impaired learning ability.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating hydroxyurea therapy.
Contraception
Females
Hydroxyurea capsules can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during and after treatment with hydroxyurea capsules for at least 6 months after therapy. Advise females to immediately report pregnancy.
Males
Hydroxyurea may damage spermatozoa and testicular tissue, resulting in possible genetic abnormalities. Males with female sexual partners of reproductive potential should use effective contraception during and after treatment with hydroxyurea capsules for at least 1 year after therapy [see Nonclinical Toxicology (13.1)].
Infertility
Males
Based on findings in animals and humans, male fertility may be compromised by treatment with hydroxyurea capsules. Azoospermia or oligospermia, sometimes reversible, has been observed in men. Inform male patients about the possibility of sperm conservation before the start of therapy [see Adverse Reactions (6) and Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
Elderly patients may be more sensitive to the effects of hydroxyurea and may require a lower dose regimen. Hydroxyurea is excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.3)].
8.6 Renal Impairment
The exposure to hydroxyurea is higher in patients with creatinine clearance of less than 60 mL/min or in patients with end-stage renal disease (ESRD). Reduce dosage and closely monitor the hematologic parameters when hydroxyurea capsule is to be administered to these patients [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
10. Overdosage
Acute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at dosages several times the therapeutic dose. Soreness, violet erythema, edema on palms and soles followed by scaling of hands and feet, severe generalized hyperpigmentation of the skin, and stomatitis have also been observed.
11. Hydroxyurea Description
Hydroxyurea capsules, USP are an antimetabolite available for oral use as capsules containing 500 mg hydroxyurea, USP. Inactive ingredients include colloidal silicon dioxide, FD&C Blue No. 1, FD&C Red No. 3, gelatin, magnesium stearate, titanium dioxide and black ink. The black ink contains black iron oxide, dewaxed shellac and potassium hydroxide.
Hydroxyurea is a white to off-white crystalline powder. It is non-hygroscopic and freely soluble in water, but practically insoluble in alcohol. The empirical formula is CH4N2O2 and it has a molecular weight of 76.05. Its structural formula is:
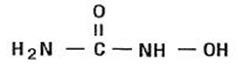
FDA approved dissolution test differs from the USP dissolution test.
12. Hydroxyurea - Clinical Pharmacology
12.1 Mechanism of Action
The precise mechanism by which hydroxyurea produces its antineoplastic effects cannot, at present, be described. However, the reports of various studies in tissue culture in rats and humans lend support to the hypothesis that hydroxyurea causes an immediate inhibition of DNA synthesis by acting as a ribonucleotide reductase inhibitor, without interfering with the synthesis of ribonucleic acid or of protein. This hypothesis explains why, under certain conditions, hydroxyurea may induce teratogenic effects.
Three mechanisms of action have been postulated for the increased effectiveness of concomitant use of hydroxyurea therapy with irradiation on squamous cell (epidermoid) carcinomas of the head and neck. In vitro studies utilizing Chinese hamster cells suggest that hydroxyurea (1) is lethal to normally radioresistant S-stage cells, and (2) holds other cells of the cell cycle in the G1 or pre-DNA synthesis stage where they are most susceptible to the effects of irradiation. The third mechanism of action has been theorized on the basis of in vitro studies of HeLa cells. It appears that hydroxyurea, by inhibition of DNA synthesis, hinders the normal repair process of cells damaged but not killed by irradiation, thereby decreasing their survival rate; RNA and protein syntheses have shown no alteration.
12.3 Pharmacokinetics
Absorption
Following oral administration of hydroxyurea capsules, hydroxyurea reaches peak plasma concentrations in 1 to 4 hours. Mean peak plasma concentrations and AUCs increase more than proportionally with increase of dose.
There are no data on the effect of food on the absorption of hydroxyurea.
Distribution
Hydroxyurea distributes throughout the body with a volume of distribution approximating total body water.
Hydroxyurea concentrates in leukocytes and erythrocytes.
Metabolism
Up to 60% of an oral dose undergoes conversion through saturable hepatic metabolism and a minor pathway of degradation by urease found in intestinal bacteria.
Excretion
In patients with sickle cell anemia, the mean cumulative urinary recovery of hydroxyurea was about 40% of the administered dose.
Specific Populations
Renal Impairment
The effect of renal impairment on the pharmacokinetics of hydroxyurea was assessed in adult patients with sickle cell disease and renal impairment. Patients with normal renal function (creatinine clearance [CrCl] >80 mL/min), mild (CrCl 50‑80 mL/min), moderate (CrCl = 30-<50 mL/min), or severe (<30 mL/min) renal impairment received a single oral dose of 15 mg/kg hydroxyurea. Patients with ESRD received two doses of 15 mg/kg separated by 7 days; the first was given following a 4-hour hemodialysis session, the second prior to hemodialysis. The exposure to hydroxyurea (mean AUC) in patients with CrCl <60 mL/min and those with ESRD was 64% higher than in patients with normal renal function (CrCl >60 mL/min). Reduce the dose of hydroxyurea capsules when it is administered to patients with creatinine clearance of <60 mL/min or with ESRD following hemodialysis [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Conventional long-term studies to evaluate the carcinogenic potential of hydroxyurea have not been performed. However, intraperitoneal administration of 125 to 250 mg/kg hydroxyurea (about 0.6-1.2 times the maximum recommended human oral daily dose on a mg/m2 basis) thrice weekly for 6 months to female rats increased the incidence of mammary tumors in rats surviving to 18 months compared to control. Hydroxyurea is mutagenic in vitro to bacteria, fungi, protozoa, and mammalian cells. Hydroxyurea is clastogenic in vitro (hamster cells, human lymphoblasts) and in vivo (SCE assay in rodents, mouse micronucleus assay). Hydroxyurea causes the transformation of rodent embryo cells to a tumorigenic phenotype.
Hydroxyurea administered to male rats at 60 mg/kg/day (about 0.3 times the maximum recommended human daily dose on a mg/m2 basis) produced testicular atrophy, decreased spermatogenesis, and significantly reduced their ability to impregnate females.
16. How is Hydroxyurea supplied
16.1 How Supplied
Hydroxyurea capsules, USP are supplied as 500 mg capsules in HDPE bottles with a child resistant cap. Each bottle contains 100 capsules. The cap is opaque powder blue and the body is opaque pink. The capsules are imprinted with “83” on the body in black ink (NDC 67184-0607-1).
16.2 Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Keep tightly closed.
16.3 Handling and Disposal
Hydroxyurea capsules are a cytotoxic drug. Follow applicable special handling and disposal procedures [see References (15)].
To decrease the risk of contact, advise caregivers to wear disposable gloves when handling hydroxyurea capsules or bottles containing hydroxyurea capsules. Wash hands with soap and water before and after contact with the bottle or capsules when handling hydroxyurea capsules. Do not open hydroxyurea capsules. Avoid exposure to crushed or opened capsules. If contact with crushed or opened capsules occurs on the skin, wash affected area immediately and thoroughly with soap and water. If contact with crushed or opened capsules occurs on the eye(s), the affected area should be flushed thoroughly with water or isotonic eyewash designated for that purpose for at least 15 minutes. If the powder from the capsule is spilled, immediately wipe it up with a damp disposable towel and discard in a closed container, such as a plastic bag; as should the empty capsules. The spill areas should then be cleaned three times using a detergent solution followed by clean water. Keep the medication away from children and pets. Contact your doctor for instructions on how to dispose of outdated capsules.
17. Patient Counseling Information
- There is a risk of myelosuppression. Monitoring blood counts weekly throughout the duration of therapy should be emphasized to patients taking hydroxyurea capsules. Advise patients to report signs and symptoms of infection or bleeding immediately [see Warnings and Precautions (5.1)].
- Advise patients of the risk of hemolytic anemia. Advise patients that they will have blood tests to evaluate for this if they develop persistent anemia [see Warnings and Precautions (5.2)].
- Advise patients that there is a risk of cutaneous vasculitic toxicities and secondary malignancies including leukemia and skin cancers [see Warnings and Precautions (5.3, 5.5)].
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy. Advise females and males of reproductive potential to use contraception during and after treatment with hydroxyurea capsules [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1, 8.3)].
- Advise patients to inform their healthcare provider if they have received or are planning to receive vaccinations while taking hydroxyurea capsules as this may result in a severe infection [see Warnings and Precautions (5.6)].
- Advise females to discontinue breastfeeding during treatment with hydroxyurea capsules [see Use in Specific Populations (8.2)].
- Patients with HIV infection should contact their physician for signs and symptoms of pancreatitis, hepatic events, and peripheral neuropathy [see Warnings and Precautions (5.7)].
- Post-irradiation erythema can occur in patients who have received previous irradiation therapy [see Warnings and Precautions (5.8)].
- Advise patients of the symptoms of potential pulmonary toxicity and instruct them to seek prompt medical attention in the event of pyrexia, cough, dyspnea, or other respiratory symptoms [see Warnings and Precautions (5.10)].
- Advise patients to notify their healthcare provider if they are using a continuous glucose monitoring system while taking hydroxyurea capsules [see Warnings and Precautions (5.11)].
| HYDROXYUREA
hydroxyurea capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Qilu Pharmaceutical Co., Ltd. (653878256) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Qilu Pharmaceutical Co., Ltd. (Biological Industrial Park) | 544532200 | manufacture(67184-0607) , analysis(67184-0607) , pack(67184-0607) | |
More about hydroxyurea
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (43)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antimetabolites
- Breastfeeding
- En español