Equaline Allergy Relief Prescribing Information
Package insert / product label
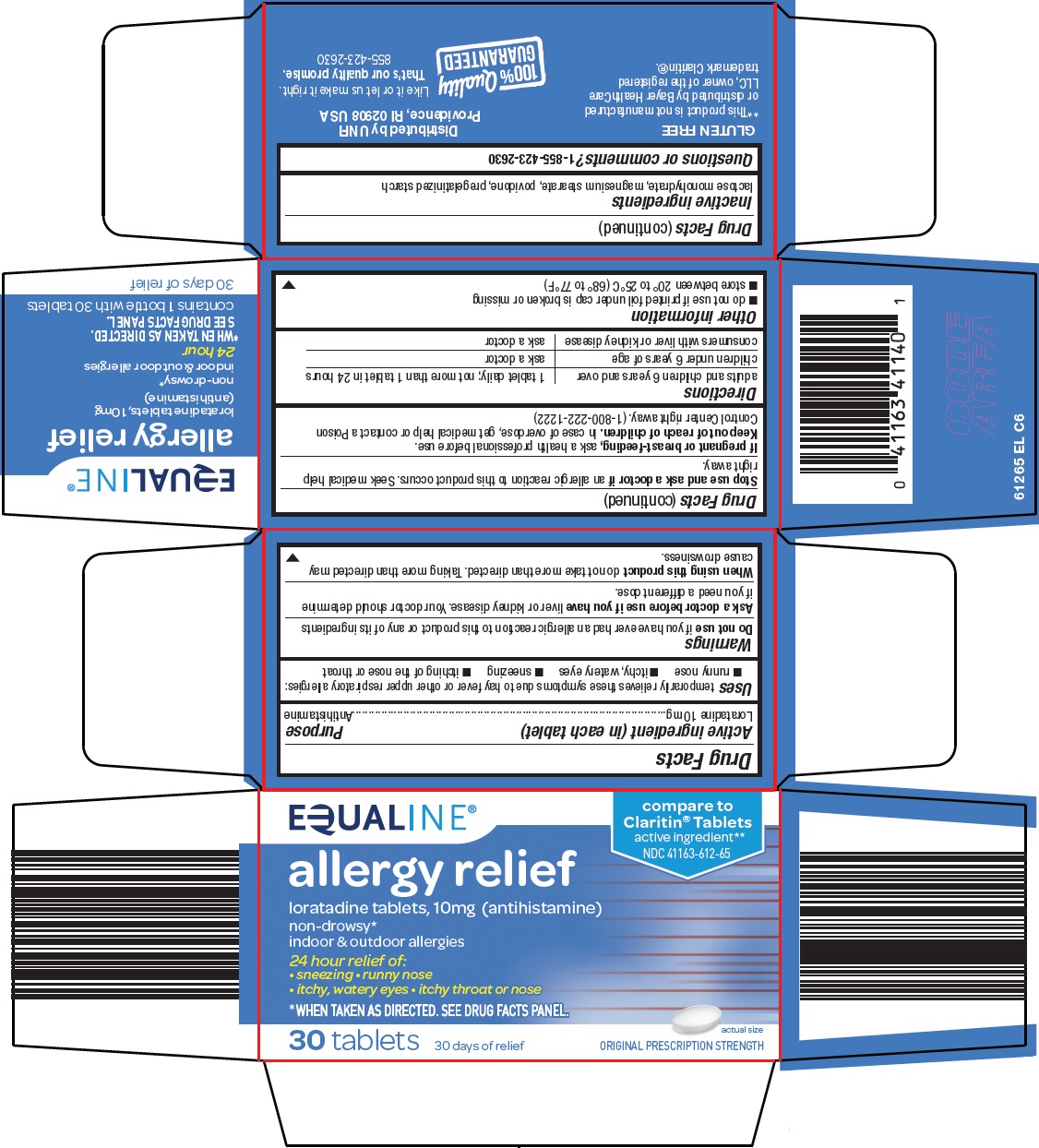
Generic name: loratadine
Dosage form: tablet
Drug class: Antihistamines
Medically reviewed by Drugs.com. Last updated on Aug 3, 2023.
Indications and Usage for Equaline Allergy Relief
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Related/similar drugs
prednisone, fluticasone nasal, montelukast, cetirizine, loratadine, promethazine, diphenhydramine
Equaline Allergy Relief Dosage and Administration
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
Storage and Handling
- •
- do not use if printed foil under cap is broken or missing
- •
- store between 20° to 25°C (68° to 77°F)
Principal Display Panel
compare to Claritin® Tablets active ingredient
allergy relief
loratadine tablets, 10mg (antihistamine)
non-drowsy*
indoor & outdoor allergies
24 hour relief of:
sneezing
runny nose
itchy, watery eyes
itchy throat or nose
*WHEN TAKEN AS DIRECTED. SEE DRUG FACTS PANEL.
30 tablets
30 days of relief
actual size
ORIGINAL PRESCRIPTION STRENGTH
| EQUALINE ALLERGY RELIEF
loratadine tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - United Natural Foods, Inc. dba UNFI (943556183) |
Frequently asked questions
- What are the most common skin conditions? (with photos)
- Can you give loratadine to dogs?
- Why do you take Claritin with Neulasta?
- Can you take 10mg of loratadine twice a day?
- Can you take antihistamines when pregnant?
More about Allergy Relief Tablets (loratadine)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: antihistamines
- Breastfeeding
Professional resources
- Topcare Allergy Relief prescribing information
- Welby Health Allergy Relief (FDA)
- Loratadine (AHFS Monograph)
Other brands
Claritin, Alavert, Allergy Relief 24 Hour, Dimetapp Children's ND Non-Drowsy Allergy