Deferiprone: Package Insert / Prescribing Info
Package insert / product label
Dosage form: tablet, coated
Drug classes: Antidotes, Chelating agents
Medically reviewed by Drugs.com. Last updated on Jun 2, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
DEFERIPRONE tablets, for oral use
Initial U.S. Approval: 2011
WARNING: AGRANULOCYTOSIS AND NEUTROPENIA
See full prescribing information for complete boxed warning.
- Deferiprone can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis. ( 5.1)
- Measure the absolute neutrophil count (ANC) before starting deferiprone and monitor regularly while on therapy. ( 5.1)
- Interrupt deferiprone therapy if neutropenia develops. ( 5.1)
- Interrupt deferiprone if infection develops and monitor the ANC more frequently. ( 5.1)
- Advise patients taking deferiprone to report immediately any symptoms indicative of infection. ( 5.1)
Recent Major Changes
Warnings and Precautions, Agranulocytosis and Neutropenia (5.1) 3/2025
Indications and Usage for Deferiprone
Deferiprone tablets are an iron chelator indicated for the treatment of transfusional iron overload in adult patients with thalassemia syndromes when current chelation therapy is inadequate. ( 1)
Limitations of Use
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
Deferiprone Dosage and Administration
- Deferiprone tablets are available in two formulations. A 1,000 mg formulation and a 500 mg formulation, which have different dosing regimens to achieve the same total daily dosage. ( 2.1)
- To prevent medication errors, before prescribing and dispensing, ensure that the tablet formulation is appropriate for the dosing regimen. Each tablet has distinct identifying characteristics. ( 2.1, 3)
- Deferiprone tablets (three times a day), 1,000 mg:
- Deferiprone tablets (three times a day), 500 mg:
Dosage Forms and Strengths
Contraindications
Hypersensitivity to deferiprone or to any of the excipients in the formulations. ( 4)
Warnings and Precautions
Adverse Reactions/Side Effects
The most common adverse reactions in patients with thalassemia (incidence > 6%) are nausea, vomiting, abdominal pain, arthralgia, ALT increased and neutropenia. (5.1, 6) (6)
(6)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc., at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch (6)
(6)
Drug Interactions
- Drugs Associated with Neutropenia or Agranulocytosis: Avoid co-administration. If co-administration is unavoidable, closely monitor the absolute neutrophil count. ( 7.1)
- UGT1A6 Inhibitors: Avoid co-administration. ( 7.2)
- Polyvalent Cations: Allow at least a 4-hour interval between administration of deferiprone and drugs or supplements containing polyvalent cations (e.g., iron, aluminum, or zinc). ( 2.6, 7.2)
Use In Specific Populations
Lactation: Advise not to breastfeed. (
8.2)
Pediatric use information is approved for Chiesi USA, Inc.'s FERRIPROX® (deferiprone) tablets. However, due to Chiesi USA, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2024
Full Prescribing Information
WARNING: AGRANULOCYTOSIS AND NEUTROPENIA
- Deferiprone can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis. [see Warnings and Precautions (5.1)]
- Measure the absolute neutrophil count (ANC) before starting deferiprone therapy and monitor regularly while on therapy.
- Interrupt deferiprone therapy if neutropenia develops. [see Warnings and Precautions (5.1)]
- Interrupt deferiprone if infection develops, and monitor the ANC more frequently. [see Warnings and Precautions (5.1)]
- Advise patients taking deferiprone to report immediately any symptoms indicative of infection. [see Warnings and Precautions (5.1)]
1. Indications and Usage for Deferiprone
Deferiprone tablets are indicated for the treatment of transfusional iron overload in adult patients with thalassemia syndromes when current chelation therapy is inadequate.
Limitations of Use
- Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
Pediatric use information is approved for Chiesi USA, Inc.'s FERRIPROX ®(deferiprone) tablets. However, due to Chiesi USA, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
2. Deferiprone Dosage and Administration
2.1 Important Dosage and Administration Information
Deferiprone tablets are available in a 1,000 mg formulation and a 500 mg formulation, which have different oral dosing regimens to achieve the same total daily dosage.
- Deferiprone tablets (three times a day) - 1,000 mg - given three times a day [see Dosage and Administration (2.3)]
- Deferiprone tablets - 500 mg - given three times a day [see Dosage and Administration (2.4)]
To prevent medication errors, before prescribing and dispensing, ensure that the tablet formulation is appropriate for the dosing regimen. Each tablet has distinct identifying characteristics [see Dosage Forms and Strengths (3)].
For patients who have trouble swallowing tablets, consider the use of oral solution (see the prescribing information for oral solution).
Monitoring for Safety
Due to the risk of agranulocytosis, monitor ANC before and during deferiprone therapy.
Test ANC prior to start of deferiprone therapy and monitor on the following schedule during treatment:
- First six months of therapy: Monitor ANC weekly;
- Next six months of therapy: Monitor ANC once every two weeks;
- After one year of therapy: Monitor ANC every two to four weeks (or at the patient's blood transfusion interval in patients that have not experienced an interruption due to any decrease in ANC [see Warnings and Precautions (5.1)] .
Due to the risk of hepatic transaminase elevations, monitor ALT before and monthly during deferiprone therapy [see Warnings and Precautions (5.2)] .
Due to the risk of zinc deficiency, monitor zinc levels before and regularly during deferiprone therapy [see Warnings and Precautions (5.3)] .
2.3 Recommended Dosage for 1,000 mg Deferiprone Tablets (three times a day) for Adult Patients with Transfusional Iron Overload due to Thalassemia Syndromes
Starting Dosage for Three Times a Day Tablets
The recommended starting oral dosage of deferiprone tablets (three times a day) is 75 mg/kg/day (actual body weight), in three divided doses per day. Table 3 describes the number of deferiprone tablets (three times a day) needed to achieve the 75 mg/kg/day total starting dosage). Round dose to the nearest 500 mg (half-tablet).
| Body Weight
(kg) | Morning | Midday | Evening |
|---|---|---|---|
| 20 | 0.5 | 0.5 | 0.5 |
| 30 | 1 | 0.5 | 1 |
| 40 | 1 | 1 | 1 |
| 50 | 1.5 | 1 | 1.5 |
| 60 | 1.5 | 1.5 | 1.5 |
| 70 | 2 | 1.5 | 2 |
| 80 | 2 | 2 | 2 |
| 90 | 2.5 | 2 | 2.5 |
To minimize gastrointestinal upset when first starting therapy, dosing can start at 45 mg/kg/day and increase weekly by 15 mg/kg/day increments until the full prescribed dose is achieved.
Dosage Adjustments for Three Times Daily Tablets
Tailor dosage adjustments for deferiprone tablets (three times a day) to the individual patient's response and therapeutic goals (maintenance or reduction of body iron burden). The maximum oral dosage is 99 mg/kg/day (actual body weight), in three divided doses per day. Table 4 describes the number of deferiprone tablets (three times a day) needed to achieve the 99 mg/day total maximum daily dosage.
| Body Weight
(kg) | Morning | Midday | Evening |
|---|---|---|---|
| 20 | 0.5 | 0.5 | 1 |
| 30 | 1 | 1 | 1 |
| 40 | 1.5 | 1 | 1.5 |
| 50 | 1.5 | 1.5 | 2 |
| 60 | 2 | 2 | 2 |
| 70 | 2.5 | 2 | 2.5 |
| 80 | 2.5 | 2.5 | 3 |
| 90 | 3 | 3 | 3 |
Pediatric use information is approved for Chiesi USA, Inc.'s FERRIPROX® (deferiprone) tablets. However, due to Chiesi USA, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
2.4 Recommended Dosage for 500 mg Deferiprone Tablets (three times a day) for Adult Patients with Transfusional Iron Overload due to Thalassemia Syndromes
Starting Dosage for Three Times a Day Tablets
The recommended starting oral dosage of deferiprone tablets (three times a day) is 75 mg/kg/day (actual body weight), in three divided doses per day. Table 5 describes the number of deferiprone tablets (three times a day) needed to achieve the 75 mg/kg/day total starting dosage. Round dose to the nearest 250 mg (half-tablet).
| Body Weight
(kg) | Morning | Midday | Evening |
|---|---|---|---|
| 20 | 1 | 1 | 1 |
| 30 | 1.5 | 1.5 | 1.5 |
| 40 | 2 | 2 | 2 |
| 50 | 2.5 | 2.5 | 2.5 |
| 60 | 3 | 3 | 3 |
| 70 | 3.5 | 3.5 | 3.5 |
| 80 | 4 | 4 | 4 |
| 90 | 4 | 4 | 4 |
To minimize gastrointestinal upset when first starting therapy, dosing can start at 45 mg/kg/day and increase weekly by 15 mg/kg/day increments until the full prescribed dose is achieved.
Dosage Adjustments
Tailor dosage adjustments for deferiprone tablets (three times a day) to the individual patient's response and therapeutic goals (maintenance or reduction of body iron burden). The maximum oral dosage is 99 mg/kg/day (actual body weight), in three divided doses per day. Table 6 describes the number of deferiprone tablets (three times a day) needed to achieve the 99 mg/day total maximum daily dosage.
| Body Weight
(kg) | Morning | Midday | Evening |
|---|---|---|---|
| 20 | 1.5 | 1 | 1.5 |
| 30 | 2 | 2 | 2 |
| 40 | 3 | 2 | 3 |
| 50 | 3.5 | 3 | 3.5 |
| 60 | 4 | 4 | 4 |
| 70 | 5 | 4.5 | 4.5 |
| 80 | 5.5 | 5 | 5.5 |
| 90 | 6 | 6 | 6 |
Pediatric use information is approved for Chiesi USA, Inc.'s FERRIPROX® (deferiprone) tablets. However, due to Chiesi USA, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
2.5 Monitoring Ferritin Levels to Assess Efficacy
Monitor serum ferritin concentration every two to three months to assess the effect of deferiprone on body iron stores. If the serum ferritin is consistently below 500 mcg/L, consider temporarily interrupting deferiprone therapy until serum ferritin rises above 500 mcg/L.
2.6 Dosage Modification for Drug Interactions
Allow at least a 4-hour interval between administration of deferiprone and other drugs or supplements containing polyvalent cations such as iron, aluminum, or zinc [see Drug Interactions (7.2), Clinical Pharmacology (12.3)] .
3. Dosage Forms and Strengths
- Tablets (three times a day): 1,000 mg are white, film-coated, oval shaped tablets; imprinted with "T" score "1 K" on one side and plain on the other side.
- Tablets: 500 mg are white to pinkish-white, capsule-shaped tablets; scored on one side, engraved "T" on the left of the score line and "5" on the right and plain on the other side.
4. Contraindications
Deferiprone is contraindicated in patients with known hypersensitivity to deferiprone or to any of the excipients in the formulations. The following reactions have been reported in association with the administration of deferiprone: Henoch-Schönlein purpura; urticaria; and periorbital edema with skin rash [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Agranulocytosis and Neutropenia
Fatal agranulocytosis can occur with deferiprone use. Deferiprone can also cause neutropenia, which may foreshadow agranulocytosis. Measure the absolute neutrophil count (ANC) before starting deferiprone therapy and monitor it regularly while on therapy [see Dosage and Administration (2.1)] .
Reduction in the frequency of ANC monitoring should be considered on an individual patient basis, according to the health care provider's assessment of the patient's understanding of the risk minimization measures required during therapy.
Interrupt deferiprone therapy if neutropenia develops (ANC < 1.5 × 10 9/L).
Interrupt deferiprone if infection develops and monitor the ANC frequently.
Advise patients taking deferiprone to immediately interrupt therapy and report to their physician if they experience any symptoms indicative of infection.
The incidence of agranulocytosis was 1% of patients in pooled clinical trials of 642 patients with thalassemia syndromes. The mechanism of deferiprone-associated agranulocytosis is unknown. Agranulocytosis and neutropenia usually resolve upon discontinuation of deferiprone, but there have been reports of agranulocytosis leading to death.
Implement a plan to monitor for and to manage agranulocytosis and neutropenia prior to initiating deferiprone treatment.
For agranulocytosis (ANC < 0.2 × 10 9 /L) and severe neutropenia (0.2 x 10 9/L ≤ ANC < 0.5 x 10 9/L):
Consider hospitalization and other management as clinically appropriate.
Do not resume deferiprone in patients who have developed agranulocytosis unless potential benefits outweigh potential risks. Do not rechallenge patients who have developed neutropenia with deferiprone unless potential benefits outweigh potential risks.
For neutropenia (ANC < 1.5 × 10 9/L and ≥ 0.5 × 10 9/L):
Instruct the patient to immediately discontinue deferiprone and all other medications with a potential to cause neutropenia.
Obtain a complete blood cell (CBC) count, including a white blood cell (WBC) count corrected for the presence of nucleated red blood cells, an absolute neutrophil count (ANC), and a platelet count daily until recovery (ANC ≥ 1.5 × 10 9/L).
5.2 Liver Enzyme Elevations
In pooled clinical trials, 7.5% of 642 patients with thalassemia syndromes treated with deferiprone developed increased ALT values. Four (0.62%) deferiprone-treated subjects discontinued the drug due to increased serum ALT levels and 1 (0.16%) due to an increase in both ALT and AST.
Monitor serum ALT values monthly during therapy with deferiprone and consider interruption of therapy if there is a persistent increase in the serum transaminase levels [see Dosage and Administration (2.1)] .
5.3 Zinc Deficiency
Decreased plasma zinc concentrations have been observed on deferiprone therapy. Monitor plasma zinc annually, and supplement in the event of a deficiency [see Dosage and Administration (2.1)].
5.4 Embryo-Fetal Toxicity
Based on findings from animal reproduction studies and evidence of genotoxicity, deferiprone can cause fetal harm when administered to a pregnant woman. The available data on the use of deferiprone in pregnant women are insufficient to inform risk. In animal studies, administration of deferiprone during the period of organogenesis resulted in embryo-fetal death and malformations at doses lower than equivalent human clinical doses. Advise pregnant women and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1)] .
Advise females of reproductive potential to use an effective method of contraception during treatment with deferiprone and for at least six months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with deferiprone and for at least three months after the last dose [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described below and elsewhere in the labeling:
- Agranulocytosis and Neutropenia [see Warnings and Precautions (5.1)]
- Liver Enzyme Elevations [see Warnings and Precautions (5.2)]
- Zinc Deficiency [see Warnings and Precautions (5.3)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reaction information represents the pooled data collected from single arm or active-controlled clinical trials with deferiprone tablets (three times a day).
Thalassemia Syndromes
The safety of deferiprone was evaluated in the pooled clinical trial database [see Clinical Studies (14.1)]. Patients received deferiprone tablets (three times a day). Deferiprone was administered orally three times a day (total daily dose either 50, 75, or 99 mg/kg), N=642. Among 642 patients receiving deferiprone, 492 (76.6%) were exposed for 6 months or longer and 365 (56.9%) were exposed for greater than one year.
The median age of patients who received deferiprone was 19 years (range 1, 77 years); 50.2% female; 71.2% White, 17.8% Asian, 9.2% Unknown, 1.2% Multi-racial and 0.6% Black.
The most serious adverse reaction reported in clinical trials with deferiprone was agranulocytosis [see Warnings and Precautions (5.1)] .
The most common adverse reactions (≥6%) reported during clinical trials were nausea, vomiting, abdominal pain, arthralgia, alanine aminotransferase increased and neutropenia.
The table below lists the adverse drug reactions that occurred in at least 1% of patients treated with deferiprone in clinical trials in patients with thalassemia syndromes.
| Body System | (N=642) |
|---|---|
| Adverse Reaction | % Patients |
| BLOOD AND LYMPHATIC SYSTEM DISORDERS | |
| Neutropenia* | 7 |
| Agranulocytosis † | 1 |
| GASTROINTESTINAL DISORDERS | |
| Nausea | 13 |
| Abdominal pain/discomfort | 10 |
| Vomiting | 10 |
| Diarrhea | 3 |
| Dyspepsia | 2 |
| INVESTIGATIONS | |
| Alanine aminotransferase increased | 7 |
| Weight increased | 2 |
| Aspartate aminotransferase increased | 1 |
| METABOLISM AND NUTRITION DISORDERS | |
| Increased appetite | 4 |
| Decreased appetite | 1 |
| MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS | |
| Arthralgia | 10 |
| Back pain | 2 |
| Pain in extremity | 2 |
| Arthropathy | 1 |
| NERVOUS SYSTEM DISORDERS | |
| Headache | 2 |
*Neutropenia includes events of severe neutropenia (ANC ≥0.2 x 10 9/L and <0.5 x 10 9/L).
†Agranulocytosis (ANC< 0.2 x 10 9/L)
Gastrointestinal symptoms such as nausea, vomiting, and abdominal pain were the most frequent adverse reactions reported by patients participating in clinical trials and led to the discontinuation of deferiprone therapy in 1.6% of patients.
Chromaturia (reddish/brown discoloration of the urine) is a result of the excretion of iron in the urine.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in patients receiving deferiprone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.
Blood and lymphatic system disorders:thrombocytosis, pancytopenia.
Cardiac disorders:atrial fibrillation, cardiac failure.
Congenital, familial and genetic disorders:hypospadias.
Eye disorders:diplopia, papilledema, retinal toxicity.
Gastrointestinal disorders:enterocolitis, rectal hemorrhage, gastric ulcer, pancreatitis, parotid gland enlargement.
General disorders and administration site conditions:chills, edema peripheral, multi-organ failure.
Hepatobiliary disorders:jaundice, hepatomegaly.
Immune system disorders:anaphylactic shock, hypersensitivity.
Infections and infestations:cryptococcal cutaneous infection, enteroviral encephalitis, pharyngitis, pneumonia, sepsis, furuncle, infectious hepatitis, rash pustular, subcutaneous abscess.
Investigations:blood bilirubin increased, blood creatinine phosphokinase increased.
Metabolism and nutrition disorders:metabolic acidosis, dehydration.
Musculoskeletal and connective tissue disorders:myositis, chondropathy, trismus.
Nervous system disorders:cerebellar syndrome, cerebral hemorrhage, convulsion, gait disturbance, intracranial pressure increased, psychomotor skills impaired, pyramidal tract syndrome, somnolence.
Psychiatric disorders:bruxism, depression, obsessive-compulsive disorder.
Renal disorders:glycosuria, hemoglobinuria.
Respiratory, thoracic and mediastinal disorders:acute respiratory distress syndrome, epistaxis, hemoptysis, pulmonary embolism.
Skin, subcutaneous tissue disorders:hyperhidrosis, periorbital edema, photosensitivity reaction, pruritis, urticaria, rash, Henoch-Schönlein purpura.
Vascular disorders:hypotension, hypertension.
Related/similar drugs
7. Drug Interactions
7.1 Drugs Associated with Neutropenia or Agranulocytosis
Avoid co-administration of deferiprone with other drugs known to be associated with neutropenia or agranulocytosis. If co-administration is unavoidable, closely monitor the absolute neutrophil count [see Warnings and Precautions (5.1)] .
7.2 Effect of Other Drugs on Deferiprone
UDP-Glucuronosyltransferases (UGT)
Avoid use of UGT1A6 inhibitors (e.g., diclofenac, probenecid, or silymarin (milk thistle)) with deferiprone [see Dosage and Administration (2), Adverse Reactions (6.1), Clinical Pharmacology (12.3)] .
Polyvalent Cations
Deferiprone has the potential to bind polyvalent cations (e.g., iron, aluminum, and zinc); allow at least a 4-hour interval between deferiprone and other medications (e.g., antacids), or supplements containing these polyvalent cations [see Dosage and Administration (2.6)] .
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
In animal reproduction studies, oral administration of deferiprone to pregnant rats and rabbits during organogenesis at doses 33% and 49%, respectively, of the maximum recommended human dose (MRHD) resulted in structural abnormalities, embryo-fetal mortality and alterations to growth (see Data) . The limited available data from deferiprone use in pregnant women are insufficient to inform a drug-associated risk of major birth defects and miscarriage. Based on evidence and developmental toxicity in animal studies, deferiprone can cause fetal harm when administered to a pregnant woman. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes .In the U.S. general population, the estimated background risk of major birth defects and of miscarriage is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Post-marketing data available from 39 pregnancies of deferiprone-treated patients and 10 pregnancies of partners of deferiprone-treated patients are as follows:
Of the 39 pregnancies in deferiprone-treated patients, 23 resulted in healthy newborns, 6 ended in spontaneous abortion, 9 had unknown outcomes, and 1 infant was born with anal atresia, nephroptosis, ventricular septal defect, hemivertebra and urethral fistula.
Of the 10 pregnancies in partners of deferiprone-treated patients, 5 resulted in healthy newborns, 1 resulted in a healthy newborn with slight hypospadias, 1 was electively terminated, 1 resulted in the intrauterine death of twins, and 2 had unknown outcomes.
Animal Data
During organogenesis, pregnant rats and rabbits received deferiprone at oral doses of 0, 30, 80 or 200 mg/kg/day, and 0, 10, 50, or 150 mg/kg/day, respectively. The daily dose was administered as two equal divided doses approximately 7 hours apart. Doses of 200 mg/kg/day in rats and 150 mg/kg/day in rabbits, approximately 33% and 49% of the MRHD, respectively, resulted in increased post-implantation loss and reduced fetal weights in the presence of maternal toxicity (reduced maternal body weight and body weight gain in both rats and rabbits; abnormal large placenta at low incidence in rats). The 200 mg/kg/day dose in rats resulted in external, visceral and skeletal fetal malformations such as cranial malformations, cleft palate, limb malrotation, anal atresia, internal hydrocephaly, anophthalmia and fused bones. The dose of 150 mg/kg/day in rabbits resulted in external fetal malformations (partially opened eyes) and minor blood vessel and skeletal variations.
In rats, malformations including micrognathia and persistent ductus arteriosus could be observed in the absence of maternal toxicity at doses equal to or greater than 30 and 80 mg/kg/day, approximately 5% and 13% of the MHRD, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of deferiprone in human milk, the effects on the breastfed child, or the effects on milk production.
Because of the potential for serious adverse reactions in the breastfed child, including the potential for tumorigenicity shown for deferiprone in animal studies, advise patients that breastfeeding is not recommended during treatment with deferiprone, and for at least 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating deferiprone.
Contraception
Females
Deferiprone can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] . Advise female patients of reproductive potential to use effective contraception during treatment with deferiprone and for at least 6 months after the last dose.
Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with deferiprone and for at least 3 months after the last dose [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
Safety and effectiveness of deferiprone tablets have not been established in pediatric patients with chronic iron overload due to blood transfusions who are less than 8 years of age.
Pediatric use information is approved for Chiesi USA, Inc.'s FERRIPROX® (deferiprone) tablets. However, due to Chiesi USA, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Clinical studies of deferiprone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
10. Overdosage
No cases of acute overdose have been reported. There is no specific antidote to deferiprone overdose.
Neurological disorders such as cerebellar symptoms, diplopia, lateral nystagmus, psychomotor slowdown, hand movements and axial hypotonia have been observed in children treated with 2.5 to 3 times the recommended dose for more than one year. The neurological disorders progressively regressed after deferiprone discontinuation.
11. Deferiprone Description
Deferiprone Tablets contain 1,000 mg or 500 mg deferiprone (3-hydroxy-1,2-dimethylpyridin-4-one), a synthetic, orally active, iron-chelating agent. The molecular formula for deferiprone is C 7H 9NO 2and its molecular weight is 139.15 g/mol. Deferiprone has the following structural formula:
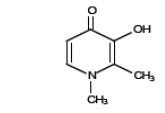
Deferiprone is a white to pinkish-white powder. It is sparingly soluble in deionized water (14.3 mg/mL) and has a melting point range of 272°C to 278°C.
Deferiprone Tablets (three times a day), 1,000 mg
White, film-coated, oval shaped tablets; imprinted with "T" score "1 K" on one side and plain on the other. The tablets can be broken in half along the score line. Each tablet contains 1,000 mg deferiprone and the following inactive ingredients: Tablet core - crospovidone, magnesium stearate and methylcellulose; Coating - copovidone, hypromellose, medium chain triglycerides, polydextrose, polyethylene glycol and titanium dioxide.
Deferiprone Tablets 500 mg
White to pinkish-white, capsule-shaped tablets; scored on one side, engraved "T" on the left of the score line and "5" on the right and plain on the other side. The tablets can be broken in half along the score line. Each tablet contains 500 mg deferiprone and the following inactive ingredients: Tablet core - colloidal silicon dioxide, magnesium stearate, and microcrystalline cellulose.
12. Deferiprone - Clinical Pharmacology
12.1 Mechanism of Action
Deferiprone is a chelating agent with an affinity for ferric ions (iron III). Deferiprone binds with ferric ions to form neutral 3:1 (deferiprone:iron) complexes that are stable at physiological pH.
12.2 Pharmacodynamics
No clinical studies were performed to assess the relationship between the dose of deferiprone and the amount of iron eliminated from the body.
12.3 Pharmacokinetics
Deferiprone Tablets (three times a day), 1,000 mg and 500 mg
The mean C maxand AUC of deferiprone was 20 mcg/mL and 50 mcg∙h/mL, respectively, in healthy subjects. The dose proportionality of deferiprone over the approved recommended dosage range is unknown.
Absorption
Deferiprone appeared in the blood within 5 to 10 minutes after oral administration. Peak serum concentration of deferiprone was reached approximately 1 to 2 hours after a single dose.
Elimination
The elimination half-life of deferiprone is approximately 2 hours.
Specific Populations
No clinically significant differences in the pharmacokinetics of deferiprone were observed based on sex, race/ethnicity, body weight, mild to severe (eGFR 15 to 89 mL/min/1.73 m 2) renal impairment, or mild (Child Pugh Class A) to moderate (Child Pugh Class B) hepatic impairment. The effect of age, including geriatric or pediatric populations, end stage renal disease or severe (Child Pugh Class C) hepatic impairment on the pharmacokinetics of deferiprone is unknown.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with deferiprone. However, in view of the genotoxicity results, and the findings of mammary gland hyperplasia and mammary gland tumors in rats treated with deferiprone in the 52-week toxicology study, tumor formation in carcinogenicity studies must be regarded as likely.
Deferiprone was positive in a mouse lymphoma cell assay in vitro. Deferiprone was clastogenic in an in vitrochromosomal aberration test in mice and in a chromosomal aberration test in Chinese Hamster Ovary cells. Deferiprone given orally or intraperitoneally was clastogenic in a bone marrow micronucleus assay in non-iron-loaded mice. A micronucleus test was also positive when mice predosed with iron dextran were treated with deferiprone. Deferiprone was not mutagenic in the Ames bacterial reverse mutation test.
A fertility and early embryonic development study of deferiprone was conducted in rats. Sperm counts, motility and morphology were unaffected by treatment with deferiprone. There were no effects observed on male or female fertility or reproductive function at the highest dose which was 25% of the MRHD.
14. Clinical Studies
The following information is based on studies with deferiprone tablets (three times a day).
14.1 Transfusional Iron Overload in Patients with Thalassemia Syndromes
In a prospective, planned, pooled analysis of patients with thalassemia syndromes from several studies, the efficacy of deferiprone was assessed in transfusion-dependent iron overload patients in whom previous iron chelation therapy had failed or was considered inadequate due to poor tolerance. The main criterion for chelation failure was serum ferritin > 2,500 mcg/L before treatment with deferiprone. Deferiprone therapy (35 to 99 mg/kg/day) was considered successful in individual patients who experienced a ≥ 20% decline in serum ferritin within one year of starting therapy.
Data from a total of 236 patients were analyzed. Of the 224 patients with thalassemia who received deferiprone monotherapy and were eligible for serum ferritin analysis, 105 (47%) were male and 119 (53%) were female. The mean age of these patients was 18.2 years (range 2 to 62; 91 patients were <17).
For the patients in the analysis, the endpoint of at least a 20% reduction in serum ferritin was met in 50% (of 236 subjects), with a 95% confidence interval of 43% to 57%.
A small number of patients with thalassemia and iron overload were assessed by measuring the change in the number of milliseconds (ms) in the cardiac MRI T2* value before and after treatment with deferiprone for one year. There was an increase in cardiac MRI T2* from a mean at baseline of 11.8 ± 4.9 ms to a mean of 15.1 ± 7.0 ms after approximately one year of treatment. The clinical significance of this observation is not known.
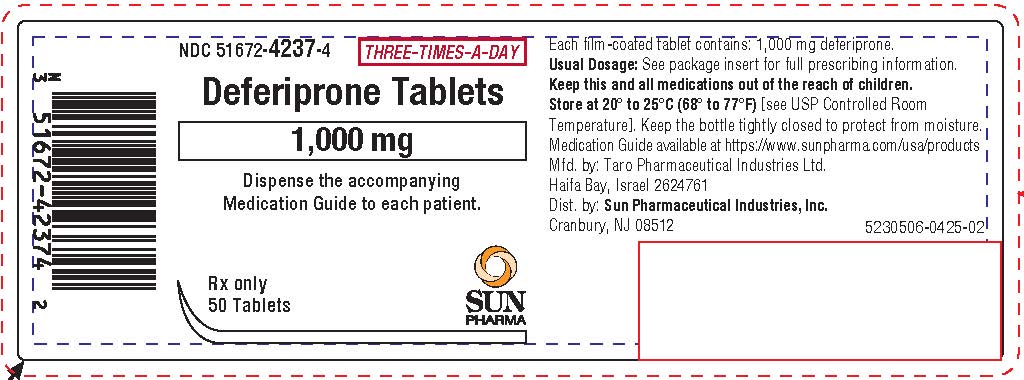
16. How is Deferiprone supplied
Deferiprone Tablets (three times a day), 1,000 mg
White, film-coated, oval shaped tablets; imprinted with "T" score "1 K" on one side and plain on the other. The tablets can be broken in half along the score line. They are provided in HDPE bottles.
1,000 mg film-coated tablets, 50 tablets
NDC 51672-4237-4
Keep the bottle tightly closed to protect from moisture.
Deferiprone Tablets, 500 mg
White to pinkish-white, capsule-shaped tablets; scored on one side, engraved "T" on the left of the score line and "5" on the right and plain on the other side. They are provided in a 100 count HDPE bottle with a child-resistant cap.
500 mg tablets, 100 tablets
NDC 51672-4196-1
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
- Instruct patients and their caregivers to store deferiprone at 68°F to 77°F (20°C to 25°C) [see USP Controlled Room Temperature].
-
Deferiprone tablets (three times a day), 1,000 mg:
Store in the originally supplied bottle, closed tightly to protect from moisture.
Advise patients to take the first dose of deferiprone in the morning, the second dose at midday, and the third dose in the evening. Clinical experience suggests that taking deferiprone with meals may reduce nausea. -
Deferiprone tablets, 500 mg:
Store in the originally supplied bottle, closed tightly to protect from moisture.
Advise patients to take the first dose of deferiprone in the morning, the second dose at midday, and the third dose in the evening. Clinical experience suggests that taking deferiprone with meals may reduce nausea. - If a dose of this medicine has been missed, take it as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to the regular dosing schedule. Do not catch-up or double doses.
- Inform patients of the risks of developing agranulocytosis and the need for regular blood testing before and during their treatment to monitor for decreases in their ANC. Instruct them to immediately interrupt therapy and report to their physician if they experience any symptoms of infection such as fever, sore throat or flu-like symptoms [see Dosage and Administration (2.1)and Warnings and Precautions (5.1)] in order to check their ANC within 24 hours. Advise them if they are unable to reach their physician, seek care from another provider so as not to delay medical care.
- Inform patients of the risk of abnormal liver transaminases and the need for regular blood testing before and during their treatment to monitor for increases in ALT [see Dosage and Administration (2.1)and Warnings and Precautions (5.2)] .
- Inform patients of the risk of zinc deficiency and the need for regular blood testing before and during their treatment to monitor for reductions in zinc [see Dosage and Administration (2.1)and Warnings and Precautions (5.3)] .
- Advise patients to contact their physician in the event of overdose.
- Inform patients that their urine might show a reddish/brown discoloration due to the excretion of the iron-deferiprone complex. This is a very common sign of the desired effect, and it is not harmful.
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4)and Use in Specific Populations (8.1)] . Advise female patients of reproductive potential to use effective contraception during treatment with deferiprone and for at least six months after the last dose [see Use in Specific Populations (8.1, 8.3)] . Advise males with female partners of reproductive potential to use effective contraception during treatment with deferiprone and for at least three months after the last dose [see Use in Specific Populations (8.3)and Nonclinical Toxicology (13.1)] .
Lactation
Advise females not to breastfeed during treatment with deferiprone and for at least 2 weeks after the last dose [see Use in Specific Populations (8.2)] .
Mfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761
Dist. by:
Sun Pharmaceutical Industries, Inc.,Cranbury, NJ 08512
Revised: April 2025
5230551-0425-03
Dispense with Medication Guide available at: https://www.sunpharma.com/usa/products
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | |||
| Medication Guide
Deferiprone (de-fer-ip-rone) Tablets |
|||
| What is the most important information I should know about deferiprone tablets?
Deferiprone tablets can cause serious side effects , including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with deferiprone tablets and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death. Your healthcare provider will do a blood test before you start deferiprone tablets and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with deferiprone tablets if you develop neutropenia or infection. Stop taking deferiprone tablets and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection:
See " What are the possible side effects of deferiprone tablets?" for more information about side effects. |
|||
| What is deferiprone?
Deferiprone is a prescription medicine used to treat iron overload from blood transfusions in adults with thalassemia syndromes when current iron removal (chelation) therapy does not work well enough. It is not known if deferiprone tablets is safe and effective to treat iron overload due to blood transfusions:
|
|||
| Do not take deferiprone tablets if you are allergic to deferiprone or any of the ingredients in deferiprone tablets.See the end of this Medication Guide for a complete list of ingredients in deferiprone tablets. | |||
Before taking deferiprone tablets, tell your healthcare provider about all of your medical conditions, including if you:
|
|||
How should I take deferiprone tablets?
|
|||
| Deferiprone Tablets
1,000 mg 3 times each day | Deferiprone Tablets
500 mg 3 times each day |
||
| Take your first dose in the morning, the second dose at midday, and the third dose in the evening. | Take your first dose in the morning, the second dose at mid-day, and the third dose in the evening. | ||
|
|||
| What are the possible side effects of deferiprone tablets?
Deferiprone tablets can cause serious side effects, including:
These are not all of the possible side effects of deferiprone tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
| How should I store deferiprone tablets? | |||
| Deferiprone Tablets
1,000 mg 3 times each day | Deferiprone Tablets
500 mg 3 times each day |
||
|
|
||
| Keep deferiprone tablets and all medicines out of the reach of children. | |||
| General information about the safe and effective use of deferiprone tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use deferiprone tablets for a condition for which it was not prescribed. Do not give deferiprone tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about deferiprone tablets that is written for health professionals. |
|||
| What are the ingredients in deferiprone tablets? | |||
| Deferiprone Tablets
1,000 mg 3 times each day | Deferiprone Tablets
500 mg 3 times each day |
||
| Active ingredient:deferiprone
Inactive ingredients:Tablet core: crospovidone, magnesium stearate and methylcellulose. Coating: copovidone, hypromellose, medium chain triglycerides, polydextrose, polyethylene glycol and titanium dioxide. | Active ingredient:deferiprone
Inactive ingredients:Tablet core: crospovidone, magnesium stearate and methylcellulose. |
||
| Mfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761
Dist. by: Sun Pharmaceutical Industries, Inc.,Cranbury, NJ 08512 For more information, call 1-866-923-4914 or visit https://www.sunpharma.com/usa/products |
|||
Pediatric use information is approved for Chiesi USA, Inc.'s FERRIPROX® (deferiprone) tablets. However, due to Chiesi USA, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
Revised: April 2025
5230551-0425-03
| DEFERIPRONE
deferiprone tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| DEFERIPRONE
deferiprone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceutical Industries, Ltd. | 600072078 | manufacture(51672-4196, 51672-4237) | |
More about deferiprone
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antidotes
- Breastfeeding
- En español

 NDC 51672-4196-1
NDC 51672-4196-1