WATSON 395 50 0 .5 Pill - green oval, 13mm
Pill with imprint WATSON 395 50 0 .5 is Green, Oval and has been identified as Naloxone Hydrochloride and Pentazocine Hydrochloride 0.5 mg / 50 mg. It is supplied by Watson Pharmaceuticals.
Naloxone/pentazocine is used in the treatment of Chronic Pain; Pain and belongs to the drug class narcotic analgesic combinations. Risk cannot be ruled out during pregnancy. Naloxone/pentazocine 0.5 mg / 50 mg is classified as a Schedule 4 controlled substance under the Controlled Substance Act (CSA).
Images for WATSON 395 50 0 .5
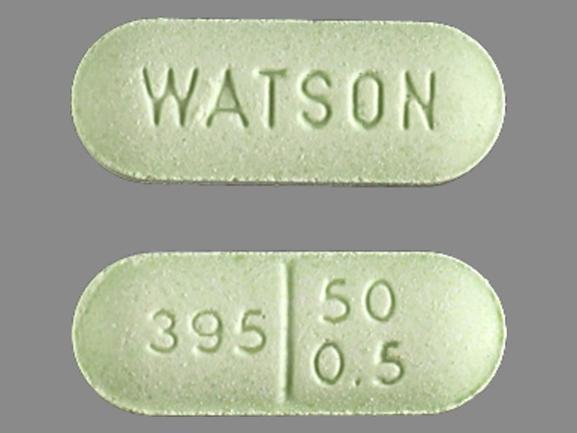

Naloxone Hydrochloride and Pentazocine Hydrochloride
- Imprint
- WATSON 395 50 0 .5
- Strength
- 0.5 mg / 50 mg
- Color
- Green
- Size
- 13.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Narcotic analgesic combinations
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- 4 - Some potential for abuse
- Labeler / Supplier
- Watson Pharmaceuticals
- Inactive Ingredients
-
silicon dioxide,
calcium phosphate dibasic anhydrous,
D&C Yellow No. 10,
FD&C Blue No. 1,
FD&C Yellow No. 6,
magnesium stearate,
microcrystalline cellulose,
corn starch,
sodium lauryl sulfate
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00591-0395 | Watson Pharmaceuticals, Inc. |
| 66267-0487 (Discontinued) | Nucare Pharmaceuticals Inc. (repackager) |
| 63874-0220 (Discontinued) | Altura Pharmaceuticals Inc. (repackager) |
| 43063-0142 (Discontinued) | PDRX Pharmaceuticals Inc. (repackager) |
| 35356-0176 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
More about naloxone / pentazocine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (26)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: narcotic analgesic combinations
- En español
Patient resources
- Naloxone and pentazocine drug information
- Pentazocine and naloxone (Advanced Reading)
- Pentazocine and Naloxone
Other brands
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
