432 ETH Pill - yellow round
Pill with imprint 432 ETH is Yellow, Round and has been identified as Ethedent (Chewable) 0.25 mg. It is supplied by Ethex Corporation.
Fluoride is used in the treatment of Prevention of Dental Caries and belongs to the drug class mouth and throat products. FDA has not classified the drug for risk during pregnancy. Fluoride 0.25 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for 432 ETH
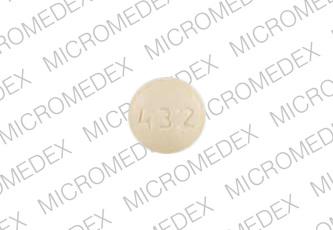
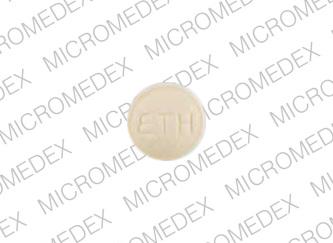

Ethedent (Chewable)
- Imprint
- 432 ETH
- Strength
- 0.25 mg
- Color
- Yellow
- Shape
- Round
- Availability
- Rx and/or OTC
- Drug Class
- Mouth and throat products
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Ethex Corporation
- National Drug Code (NDC)
- 58177-0432 (Discontinued)
More about fluoride topical
- Compare alternatives
- Pricing & coupons
- Reviews (32)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: mouth and throat products
- En español
Patient resources
- Fluoride topical drug information
- Sodium fluoride (Advanced Reading)
- Sodium Fluoride Cream, Gel, and Paste
- Sodium Fluoride Rinse
- Stannous Fluoride Gel
- Stannous Fluoride Rinse
Other brands
Prevident, Biotene, Denta 5000 Plus, Neutral Sodium Fluoride Rinse, ... +14 more
Professional resources
- 60 Second Fluoride Gel prescribing information
- Fluoride Foam (FDA)
- Sodium Fluoride Dental Cream (FDA)
- Sodium Fluoride Dental Gel (FDA)
- Sodium Fluoride Paste (FDA)
Other brands
Prevident 5000 Plus, Fluoridex, Clinpro 5000, PreviDent 5000 Booster, ... +37 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
