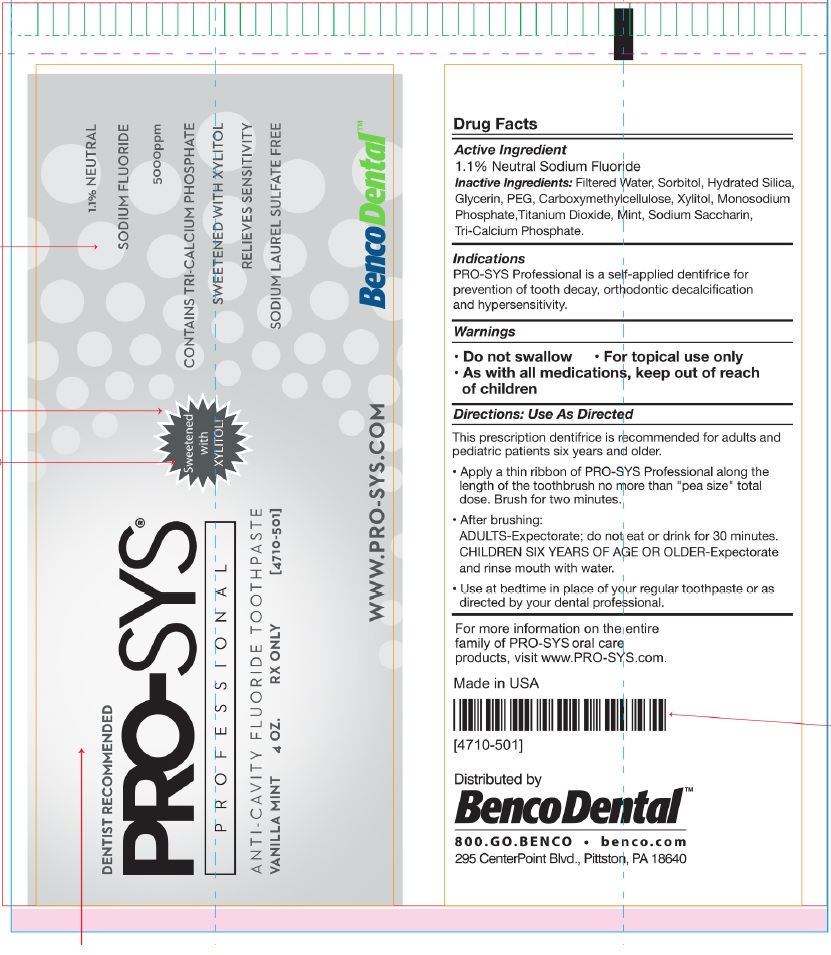
ProSys 5000 Prescribing Information
Package insert / product label
Generic name: sodium fluoride
Dosage form: paste, dentifrice
Drug class: Mouth and throat products
Medically reviewed by Drugs.com. Last updated on Nov 5, 2023.
On This Page
Inactive Ingredients:Filtered Water, Sorbitol, Hydrated Silica,
Glycerin, PEG, Carboxymethylcellulose, Xylitol, Monosodium
Phosphate,Titanium Dioxide, Mint, Sodium Saccharin,
Tri-Calcium Phosphate.
Indications
PRO-SYS Professional is a self-applied dentifrice for - prevention of tooth decay, orthodontic decalcification - and hypersensitivity
Warnings
• Do not swallow • For topical use only
• As with all medications, keep out of reach
of children
Directions: Use As Directed
This prescription dentifrice is recommended for adults and
pediatric patients six years and older.
• Apply a thin ribbon of PRO-SYS Professional along the
length of the toothbrush no more than "pea size" total
dose. Brush for two minutes.
• After brushing:
ADULTS-Expectorate; do not eat or drink for 30 minutes.
CHILDREN SIX YEARS OF AGE OR OLDER-Expectorate
and rinse mouth with water.
• Use at bedtime in place of your regular toothpaste or as
directed by your dental professional.
| PROSYS 5000
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Benco Dental (015108087) |
| Registrant - Benco Dental (015108087) |
More about ProSys 5000 (fluoride topical)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: mouth and throat products
Professional resources
Other brands
Prevident 5000 Plus, Clinpro 5000, PreviDent 5000 Booster, Ionite APF Gel, ... +36 more