Hizentra Dosage
Generic name: HUMAN IMMUNOGLOBULIN G 0.2g in 1mL
Dosage form: subcutaneous infusion
Drug class: Immune globulins
Medically reviewed by Drugs.com. Last updated on Apr 10, 2025.
For subcutaneous infusion only.
Preparation and Handling
HIZENTRA is a clear and pale yellow to light brown solution. Do not use if the solution is cloudy or contains particulates.
- Prior to administration, visually inspect each prefilled syringe or vial of HIZENTRA for particulate matter or discoloration, whenever the solution and container permit.
- Do not freeze. Do not use any solution that has been frozen.
- Check the product expiration date on the prefilled syringe or vial label. Do not use beyond the expiration date.
- Do not mix HIZENTRA with other products.
- Do not shake the prefilled syringe or vial.
- Use aseptic technique when preparing and administering this product.
- Both the HIZENTRA prefilled syringe and vial are single-dose containers. Multiple HIZENTRA prefilled syringes or vials can be administered to achieve the prescribed dose. Discard all used administration supplies and any unused product immediately after each infusion in accordance with local requirements.
Dose
Primary Immunodeficiency (PI)
- HIZENTRA can be administered at regular intervals from daily up to every 2 weeks (biweekly).
- Individualize the dose based on the patient's clinical response to HIZENTRA therapy and serum immunoglobulin G (IgG) trough levels.
- Before receiving treatment with HIZENTRA:
- Ensure that patients have received Immune Globulin Intravenous (Human) (IGIV) treatment at regular intervals for at least 3 months.
- Obtain the patient's serum IgG trough level to guide subsequent dose adjustments (see below, under Dose Adjustment).
Dosage for patients switching to HIZENTRA from IGIV
- Establish the initial weekly dose of HIZENTRA by converting the monthly IGIV dose into a weekly equivalent and increasing it using the dose adjustment factor. The goal is to achieve a systemic serum IgG exposure (area under the concentration-time curve [AUC]) not inferior to that of the previous IGIV treatment.
- To calculate the initial weekly dose of HIZENTRA, divide the previous IGIV dose in grams by the number of weeks between doses during the patient's IGIV treatment (e.g., 3 or 4); then multiply this by the dose adjustment factor of 1.37.
Initial HIZENTRA dose = Previous IGIV dose (in grams) × 1.37 Number of weeks between IGIV doses - To convert the HIZENTRA dose (in grams) to milliliters (mL), multiply the calculated dose (in grams) by 5.
- To calculate the initial weekly dose of HIZENTRA, divide the previous IGIV dose in grams by the number of weeks between doses during the patient's IGIV treatment (e.g., 3 or 4); then multiply this by the dose adjustment factor of 1.37.
- Provided the total weekly dose is maintained, any dosing interval from daily up to biweekly can be used and will result in systemic serum IgG exposure that is comparable to the previous IGIV or weekly HIZENTRA treatment.
- For biweekly dosing, multiply the calculated HIZENTRA weekly dose by 2.
- For frequent dosing (2 to 7 times per week), divide the calculated weekly dose by the desired number of times per week (e.g., for 3 times per week dosing, divide weekly dose by 3).
Dosage for patients switching to HIZENTRA from IGSC
- The previous weekly IGSC dose should be maintained.
- For biweekly dosing, multiply the previous weekly dose by 2.
- For frequent dosing (2 to 7 times per week), divide the previous weekly dose by the desired number of times per week (e.g., for 3 times per week dosing, divide weekly dose by 3).
Start HIZENTRA treatment
- For weekly or frequent dosing, start treatment with HIZENTRA 1 week after the patient's last IGIV infusion or IGSC infusion.
- For biweekly dosing, start treatment 1 or 2 weeks after the last IGIV infusion or 1 week after the last weekly IGSC infusion.
Dose Adjustment
The dose may need to be adjusted to achieve the desired clinical response and serum IgG trough level, irrespective of the frequency of administration.
To determine if a dose adjustment should be considered, measure the patient's serum IgG trough level 2 to 3 months after switching to HIZENTRA.
Weekly dosing: When switching from IGIV to weekly HIZENTRA dosing, the target serum IgG trough level is projected to be approximately 16% higher than the last trough level during prior IGIV therapy.
Biweekly dosing: When switching from IGIV to biweekly HIZENTRA dosing, the target serum IgG trough level is projected to be approximately 10% higher than the last IGIV trough level. When switching from weekly to biweekly dosing, the target trough is projected to be approximately 5% lower than the last trough level on weekly therapy.
Frequent dosing: When switching from weekly dosing to more frequent dosing, the target serum IgG trough level is projected to be approximately 3 to 4% higher than the last trough level on weekly therapy.
To adjust the dose based on serum trough levels, calculate the difference (in mg/dL) between the patient's IgG trough level obtained 2 to 3 months following the switch from IGIV or the last IGSC dose adjustment and the target IgG trough level for weekly or biweekly dosing. Then find this difference in Table 1 (Column 1) and, based on the HIZENTRA dosing frequency (for weekly or biweekly) and the patient's body weight, locate the corresponding adjustment amount (in mL) by which to increase (or decrease) the dose. For frequent dosing, add the weekly increment from Table 1 to the weekly-equivalent dose and then divide by the number of days of dosing.
Use the patient's clinical response as the primary consideration in dose adjustment. Additional dosage increments may be indicated based on the patient's clinical response (infection frequency and severity).
| Difference From Target Serum IgG Trough Level (mg/dL) | Dosing Frequency | Weight Adjusted Dose Increment (mL)* | ||||
|---|---|---|---|---|---|---|
| Weight Group | ||||||
| >10 to ≤30 kg | >30 to ≤50 kg | >50 to ≤70 kg | >70 to ≤90 kg | >90 kg | ||
| n/a, not applicable. | ||||||
|
||||||
| 50 | Weekly‡ | n/a | 2.5 | 5 | 5 | 10 |
| Biweekly | 5 | 5 | 10 | 10 | 20 | |
| 100 | Weekly‡ | 2.5 | 5 | 10 | 10 | 15 |
| Biweekly | 5 | 10 | 20 | 20 | 30 | |
| 200 | Weekly‡ | 5 | 10 | 15 | 20 | 30 |
| Biweekly | 10 | 20 | 30 | 40 | 60 | |
For example, if a patient with a body weight of 70 kg has an actual IgG trough level of 900 mg/dL and the target trough level is 1000 mg/dL, this results in a difference of 100 mg/dL. Therefore, increase the weekly dose of HIZENTRA by 10 mL. For biweekly dosing, increase the biweekly dose by 20 mL. For 2 times per week dosing, increase the dose by 5 mL.
Monitor the patient's clinical response, and repeat the dose adjustment as needed.
Dosage requirements for patients switching to HIZENTRA from another IGSC product: If a patient on HIZENTRA does not maintain an adequate clinical response or a serum IgG trough level equivalent to that of the previous IGSC treatment, the physician may want to adjust the dose. For such patients, Table 1 also provides guidance for dose adjustment if their desired IGSC trough level is known.
Measles Exposure
Administer a minimum total weekly HIZENTRA dose of 0.2 g/kg body weight for 2 consecutive weeks if a patient is at risk of measles exposure (i.e., due to an outbreak in the U.S. or travel to endemic areas outside of the U.S.). For biweekly dosing, one infusion of a minimum at 400 mg/kg is recommended. If a patient has been exposed to measles, ensure this minimum dose is administered as soon as possible after exposure.
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Initiate therapy with HIZENTRA 1 week after the last IGIV infusion.
- The recommended subcutaneous dose is 0.2 g/kg (1 mL/kg) body weight per week, administered in 1 or 2 sessions over 1 or 2 consecutive days.
- -
- In the clinical study after transitioning from IGIV to HIZENTRA treatment, a dose of 0.4 g/kg (2 mL/kg) body weight per week was also safe and effective in preventing CIDP relapse.
- If CIDP symptoms worsen on 0.2 g/kg (1 mL/kg) body weight per week, consider increasing the HIZENTRA dose from 0.2 g/kg (1 mL/kg) to 0.4 g/kg (2 mL/kg) body weight per week, administered in 2 sessions per week over 1 or 2 consecutive days.
- -
- If CIDP symptoms worsen on the 0.4 g/kg body weight per week dose, consider re-initiating therapy with an IGIV product approved for treatment of CIDP, while discontinuing HIZENTRA.
- Monitor the patient's clinical response and adjust the duration of therapy based on patient need.
Administration
HIZENTRA is for subcutaneous infusion only.
HIZENTRA is intended for subcutaneous administration using an infusion pump. Infuse HIZENTRA in the abdomen, thigh, upper arm, and/or lateral hip.
- Infusion sites – A HIZENTRA dose may be infused into multiple infusion sites. Use up to 8 infusion sites in parallel. More than one infusion device can be used simultaneously. Infusion sites should be at least 2 inches apart. Change the actual site of infusion with each administration.
- Volume (as tolerated) – For the first infusion of HIZENTRA, do not exceed a volume of 15 mL per infusion site in patients with PI or up to 20 mL per infusion site in patients with CIDP. For subsequent infusions, the volume may be increased to 25 mL per infusion site for patients with PI or to 50 mL per site for patients with CIDP.
- Rate (as tolerated) – For the first infusion of HIZENTRA, the recommended flow rate is up to 15 mL per hour per infusion site in patients with PI or up to 20 mL per hour per site in patients with CIDP. For subsequent infusions, the flow rate may be increased to 25 mL per hour per site in patients with PI or up to 50 mL per hour per site in patients with CIDP.
Follow the steps below and use aseptic technique to administer HIZENTRA, either as prefilled syringe(s) or vial(s).
- 1.
- Assemble supplies Gather the HIZENTRA prefilled syringe(s) or vial(s), all supplies, and infusion log book.
- 2.
- Clean surface Clean a table or other flat surface.
- 3.
- Wash hands – Thoroughly wash and dry hands (Figure 1).

Figure 1
- 4.
- Check prefilled syringe(s) or vial(s):
- If using prefilled syringes, carefully peel back the transparent covering from the tray and inspect the protective cap. Peel back the outer layer of the wrap-around label to allow for viewing of HIZENTRA through the fully transparent inner layer, but don't remove the label completely (Figure 2).

Figure 2
- If using vials, inspect the protective cap of the vials (Figure 3).
Figure 3
- If using prefilled syringes, carefully peel back the transparent covering from the tray and inspect the protective cap. Peel back the outer layer of the wrap-around label to allow for viewing of HIZENTRA through the fully transparent inner layer, but don't remove the label completely (Figure 2).
Carefully inspect each prefilled syringe(s) or vial(s) of HIZENTRA. HIZENTRA is a clear and pale yellow to light brown solution. Do not use the prefilled syringe or vial if damaged, the liquid looks cloudy, contains particles, has changed color, the protective cap of the prefilled syringe or the vial is missing or defective, or the expiration date on the label has passed.
- 5.
- Prepare HIZENTRA for infusion
If using HIZENTRA prefilled syringes, go to Step 5.1
If using HIZENTRA vials, go to Step 5.2 - 5.1
- HIZENTRA prefilled syringe(s)
- The 5 mL, 10 mL, 20 mL, and 50 mL prefilled syringes are supplied and ready to use. The 5 mL and 10 mL prefilled syringes are fully assembled (Figure 4). For the 20 mL and 50 mL prefilled syringes, screw the plunger rod onto the prefilled syringe stopper prior to use (Figure 5).

Figure 4

Figure 5
- HIZENTRA prefilled syringes can be placed directly in the infusion pump if the prefilled syringe size matches the pump requirements. If the prefilled syringe can be placed directly in the infusion pump, then go to Step 6.
NOTE:
An additional adapter may be required for the HIZENTRA prefilled syringes to fit properly in the infusion pump. Check with the provider of your supplies for the appropriate adapter and installation instructions. - If the HIZENTRA prefilled syringe size does not match the infusion pump requirements, transfer the contents of the prefilled syringe to another syringe of a size specific for the infusion pump by following the directions below:
- Use a syringe-to-syringe transfer device (tip-to-tip connector) (Figure 6) to transfer the contents of the prefilled syringe to the empty syringe specific for the infusion pump.

Figure 6
- Remove the protective cap from the prefilled syringe. Attach the transfer device (tip-to-tip connector) by twisting it onto the prefilled syringe. Attach the empty syringe by screwing it onto the other side of the transfer device (Figure 7).

Figure 7
- Make sure you transfer the amount needed to achieve the prescribed dose.
- Measure dose from the top of the stopper
- Push the plunger of the prefilled syringe (e.g., using your fingers, thumb or the palm of your hand) to transfer HIZENTRA from the prefilled syringe to the empty syringe.
- Repeat this step if multiple prefilled syringes are necessary to achieve the prescribed dose. Remove the emptied prefilled syringe and attach another prefilled syringe to the transfer device (tip-to-tip connector).
- After the transfer is complete, remove the emptied prefilled syringe and the transfer device (tip-to-tip connector) by unscrewing them from the syringe specific for your pump. Connect the filled syringe to the infusion tubing.
- Use a syringe-to-syringe transfer device (tip-to-tip connector) (Figure 6) to transfer the contents of the prefilled syringe to the empty syringe specific for the infusion pump.
- The 5 mL, 10 mL, 20 mL, and 50 mL prefilled syringes are supplied and ready to use. The 5 mL and 10 mL prefilled syringes are fully assembled (Figure 4). For the 20 mL and 50 mL prefilled syringes, screw the plunger rod onto the prefilled syringe stopper prior to use (Figure 5).
Go to Step 6.
- 5.2
- Transfer HIZENTRA from vial to syringe
- Take the protective cap off the vial (Figure 8).

Figure 8
- Clean the vial stopper with an alcohol wipe (Figure 9). Let the stopper dry.

Figure 9
- If using a transfer device, follow the instructions provided by the device manufacturer.
- If using a needle and a syringe to transfer HIZENTRA, follow the instructions below:
- Attach a sterile transfer needle to a sterile syringe (Figure 10).
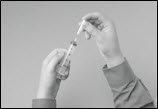
Figure 10
- Pull out the plunger of the syringe to fill the syringe with air. Make sure that the amount of air is the same as the amount of HIZENTRA you will transfer from the vial.
- Put the HIZENTRA vial on a flat surface. Keeping the vial upright, insert the transfer needle into the center of the rubber stopper.
- Check that the tip of the needle is not in the liquid. Then, push the plunger of the syringe down. This will inject the air from the syringe into the airspace of the vial.
- Leaving the needle in the stopper, carefully turn the vial upside down (Figure 11).
Figure 11
- Slowly pull back on the plunger of the syringe to fill the syringe with HIZENTRA.
- Take the filled syringe and needle out of the stopper.
- Take off the needle and throw it away in the sharps container.
- Attach a sterile transfer needle to a sterile syringe (Figure 10).
- Take the protective cap off the vial (Figure 8).
When using multiple vials to achieve the prescribed dose, repeat this step.
- 6.
- Prepare infusion pump and tubing
- Prior to preparing the pump(s), make sure you have the prescribed dose in the syringe(s) and the infusion tubing is attached.
- Prepare the infusion pump following the manufacturer's instructions, including attaching any necessary adapters.
- Do not remove the needle caps until you are ready to infuse.
- Prime (fill) the infusion. To prime the tubing, connect the syringe filled with HIZENTRA to the infusion tubing and gently push on the syringe plunger (e.g., using your fingers or thumb or the palm of your hand) to fill the tubing with HIZENTRA (Figure 12).
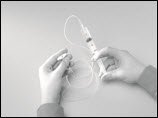
Figure 12
- Stop priming before HIZENTRA fluid reaches the capped needle.
- Insert the syringe filled with HIZENTRA into the infusion pump.
- Prime (fill) the infusion. To prime the tubing, connect the syringe filled with HIZENTRA to the infusion tubing and gently push on the syringe plunger (e.g., using your fingers or thumb or the palm of your hand) to fill the tubing with HIZENTRA (Figure 12).
- 7.
- Prepare infusion site(s)
- Select an area on your abdomen, thigh, upper arm, or side of upper leg/hip for the infusion (Figure 13).The number and location of infusion sites depends on the volume of the total dose.

Figure 13
- Never infuse into areas where the skin is tender, bruised, red, or hard. Avoid infusing into scars or stretch marks.
- Infuse HIZENTRA into a maximum of 8 sites simultaneously; or up to 12 consecutively per infusion. Infusion sites should be at least 2 inches apart.
- Clean the skin at each infusion site with an antiseptic skin prep (Figure 14). Let the skin dry.
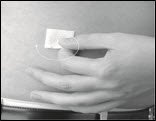
Figure 14
- Select an area on your abdomen, thigh, upper arm, or side of upper leg/hip for the infusion (Figure 13).The number and location of infusion sites depends on the volume of the total dose.
- 8.
- Insert needle(s)
- Use just one needle per site. If injecting in more than one site, the following steps should be completed for each needle, one at a time:
- Remove the needle cap.
- Using 2 fingers, pinch together the skin around the infusion site. With a quick dart-like motion, insert the needle straight into the skin (Figure 15).
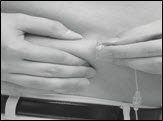
Figure 15
- Put sterile gauze and tape or a transparent dressing over the infusion site (Figure 16) to hold the needle in place.
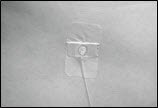
Figure 16
- 9.
- Start infusion
- Follow the manufacturer's instructions to turn on the infusion pump and start the infusion (Figure 17).

Figure 17
- Follow the manufacturer's instructions to turn on the infusion pump and start the infusion (Figure 17).
- 10.
- Complete infusion and record treatment (Figure 18)
- When all the HIZENTRA has been infused, turn off the infusion pump.
- Remove the needle(s) from the skin.
- Once you have removed the needle(s), remove the empty syringe from the infusion pump.
- Disconnect the infusion set from the empty syringe.
- Cover the infusion site(s) with a protective dressing.
- Peel-off the removable part of the label for each prefilled syringe or vial used Affix it to the patient's log book with the date and time of infusion, or scan the prefilled syringe or vial if recording the infusion electronically.

Figure 18
- 11.
- Clean up
- If applicable, remove adapter from the infusion pump following the manufacturer's instructions.
- Throw away the empty HIZENTRA prefilled syringe(s) or vial(s), along with the used disposable supplies in the sharps container (Figure 19) in accordance with local requirements.
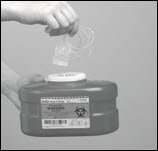
Figure 19
- Clean and store the infusion pump, following the manufacturer's instructions.
For self-administration, provide the patient with instructions and training for subcutaneous infusion in the home or other appropriate setting.
More about Hizentra (immune globulin subcutaneous)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (35)
- Latest FDA alerts (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: immune globulins
- En español
Patient resources
Other brands
Cutaquig, Xembify, Cuvitru, Vivaglobin
Professional resources
Other brands
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.




















