Abstral Sublingual Tablet Dosage
Generic name: fentanyl citrate 100ug
Dosage form: sublingual tablet
Drug class: Opioids (narcotic analgesics)
Medically reviewed by Drugs.com. Last updated on Dec 6, 2023.
Important Dosage and Administration Information
- Healthcare professionals who prescribe ABSTRAL on an outpatient basis must enroll in the TIRF REMS Access program and comply with the requirements of the REMS to ensure safe use of ABSTRAL [see Warnings and Precautions (5.7)].
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
- It is important to minimize the number of strengths available to patients at any time to prevent confusion and possible overdose.
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.6)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with ABSTRAL; adjust the dosage accordingly [see Warnings and Precautions (5.1)].
- Instruct patients and caregivers to take steps to store ABSTRAL securely and to properly dispose of unused ABSTRAL as soon as no longer needed [see Warnings and Precautions (5.2, 5.6), Patient Counseling Information (17)].
- ABSTRAL is not bioequivalent with other fentanyl products. Do not convert patients on a mcg per mcg basis from other fentanyl products. There are no conversion directions available for patients on any other fentanyl products other than ACTIQ (Note: This includes oral, transdermal, or parenteral formulations of fentanyl.) [see Warnings and Precautions (5.5)].
- ABSTRAL is NOT a generic version of any other oral transmucosal fentanyl product.
Initial Dosage
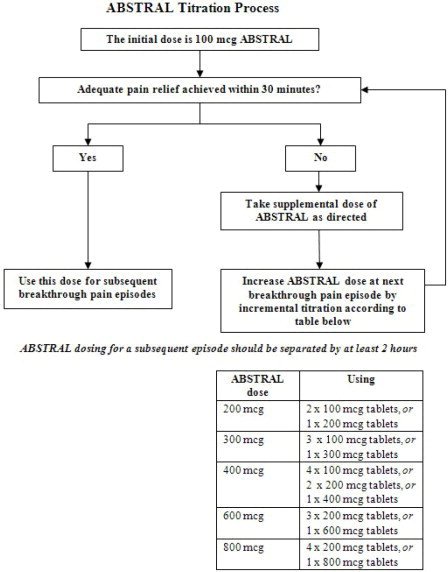
Initiate treatment with ABSTRAL for all patients with a single initial dose of 100 mcg. The initial dose of ABSTRAL is always 100 mcg, with the only exception being patients already using ACTIQ.
- If adequate analgesia is obtained within 30 minutes of administration of the 100 mcg tablet, continue to treat subsequent episodes of breakthrough pain with this dose.
- If adequate analgesia is not obtained after a single dose of ABSTRAL, the patient may use a second ABSTRAL dose (after 30 minutes) as directed by their healthcare provider. No more than two doses of ABSTRAL may be used to treat an episode of breakthrough pain [see Titration and Maintenances of Therapy(2.3)].
- Patients must wait at least 2 hours before treating another episode of breakthrough pain with ABSTRAL.
Due to differences in the pharmacokinetic properties and individual variability, even patients switching from other products containing fentanyl to ABSTRAL must start with the 100 mcg dose (except patients switching from ACTIQ).
ABSTRAL is not equivalent on a mcg per mcg basis with all other fentanyl products. Therefore, do not switch patients on a mcg per mcg basis from any other fentanyl products. ABSTRAL is NOT a generic version of any other fentanyl product.
Converting to ABSTRAL from ACTIQ
- For patients being converted from ACTIQ, prescribers must use the Initial Dosing Recommendations for Patients on ACTIQ. See Table 1 for initial dosing recommendations. Patients must be instructed to stop the use of ACTIQ and dispose of any remaining units.
Table 1: Initial Dosing Recommendations for Patients on ACTIQ Current ACTIQ Dose (mcg) Initial ABSTRAL Dose (mcg) 200 100 400 200 600 200 800 200 1200 200 1600 400 - For patients converting from ACTIQ doses of 200 mcg and 400 mcg, initiate titration with 100 mcg and 200 mcg of ABSTRAL, respectively and proceed using multiples of this strength.
- For patients converting from ACTIQ doses of 600 and 800 mcg, initiate titration with 200 mcg of ABSTRAL and proceed using multiples of this strength.
- For patients converting from ACTIQ doses of 1200 and 1600 mcg, initiate titration with 200 mcg and 400 mcg of ABSTRAL, respectively and proceed using multiples of this strength.
Titration and Maintenance of Therapy
Titration
The objective of dose titration is to identify an effective and tolerable maintenance dose. From an initial dose, closely follow patients and change the dosage strength until the patient reaches a dose that provides adequate analgesia using a single ABSTRAL dosage unit per breakthrough cancer pain episode. If signs of excessive opioid effects appear before the unit is consumed, the dosage unit should be removed from the patient's mouth immediately, disposed of properly, and subsequent doses should be decreased. Patients should record their use of ABSTRAL over several episodes of breakthrough cancer pain and review their experience with their healthcare providers to determine if a dosage adjustment is warranted for management of breakthrough cancer pain episodes. The effective and tolerable dose of ABSTRAL will be determined by dose titration in individual patients.
If adequate analgesia was not obtained with the first 100 mcg dose, continue dose escalation in a stepwise manner over consecutive breakthrough episodes until adequate analgesia with tolerable side effects is achieved. Increase the dose by 100 mcg multiples up to 400 mcg as needed. If adequate analgesia is not obtained with a 400 mcg dose, the next titration step is 600 mcg. If adequate analgesia is not obtained with a 600 mcg dose, the next titration step is 800 mcg. During titration, patients can be instructed to use multiples of 100 mcg tablets and/or 200 mcg tablets for any single dose. Instruct patients not to use more than 4 tablets at one time. If adequate analgesia is not obtained 30 minutes after the use of ABSTRAL, the patient may repeat the same dose of ABSTRAL. No more than two doses of ABSTRAL may be used to treat an episode of breakthrough pain. Rescue medication, as directed by the health care provider, can be used if adequate analgesia is not achieved after use of ABSTRAL.
The efficacy and safety of doses higher than 800 mcg have not been evaluated in clinical studies in patients.
In order to minimize the risk of ABSTRAL-related adverse reactions and to identify the appropriate dose, it is imperative that patients be supervised closely by health professionals during the titration process.
Maintenance Therapy
Once titrated to an effective dose, instruct patients to use only one ABSTRAL tablet of the appropriate strength per dose. Maintain patients on this dose.
If adequate analgesia is not obtained after initial dose of ABSTRAL, the patient may use a second ABSTRAL dose (after 30 minutes), as directed by their healthcare provider. No more than two doses of ABSTRAL may be used to treat an episode of breakthrough pain.
Patients must wait at least 2 hours before treating another episode of breakthrough pain with ABSTRAL.
Dose Re-Adjustment
If the response (analgesia or adverse reactions) to the titrated ABSTRAL dose markedly changes, an adjustment of dose may be necessary to ensure that an appropriate dose is maintained. If the level of pain increases after dosage stabilization, attempt to identify the source of increased pain before increasing the ABSTRAL dosage. If unacceptable opioid-related adverse reactions are observed, consider reducing the dosage. Adjust the dosage to obtain an appropriate balance between management of breakthrough pain and opioid-related adverse reactions.
If more than four episodes of breakthrough pain are experienced per day, re-evaluate the dose of the long- acting opioid used for persistent underlying cancer pain. If the long-acting opioid or dose of long-acting opioid is changed, re-evaluate and re-titrate the ABSTRAL dose, as necessary, to ensure the patient is on an appropriate dose.
Limit the use of ABSTRAL to treat four or fewer episodes of breakthrough pain per day.
It is imperative that any dose re-titration is monitored carefully by a healthcare professional.
Administration of ABSTRAL
Instruct patients to place ABSTRAL tablets on the floor of the mouth, directly under the tongue, immediately after removal from the blister unit and not to chew, suck, or swallow ABSTRAL tablets. Allow ABSTRAL tablets to completely dissolve in the sublingual cavity. Advise patients not to eat or drink anything until the tablet is completely dissolved.
In patients who have a dry mouth, water may be used to moisten the buccal mucosa before taking ABSTRAL.
Discontinuation of ABSTRAL
For patients no longer requiring opioid therapy, consider discontinuing ABSTRAL, along with a gradual downward titration of other opioids to minimize possible withdrawal effects.
In patients who continue to take their chronic opioid therapy for persistent pain, but no longer require treatment for breakthrough pain, ABSTRAL therapy can usually be discontinued immediately.
Disposal of ABSTRAL
Patients and their household members must be advised to dispose of any tablets remaining from a prescription as soon as they are no longer needed. Instructions are included in Patient Counseling Information (17) and in the Medication Guide.
To dispose of any unused ABSTRAL tablets, remove them from the blister cards and flush them down the toilet. Do not dispose of the ABSTRAL blister cards or cartons down the toilet.
If additional assistance is required, call 1-888-227-8725.
Frequently asked questions
- Which drugs cause opioid-induced constipation?
- Which painkiller should you use?
- Why is fentanyl so dangerous?
- What are the symptoms of a fentanyl overdose?
- Fentanyl test strips: where to get & how to use?
- How long does Fentanyl stay in your system?
- Carfentanil vs Fentanyl: Which is more dangerous?
- How does fentanyl compare to heroin or other opiates?
More about Abstral (fentanyl)
- Check interactions
- Compare alternatives
- Reviews (2)
- Imprints, shape & color data
- Latest FDA alerts (14)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: Opioids (narcotic analgesics)
- Breastfeeding
Patient resources
Other brands
Duragesic, Fentanyl Transdermal System, Sublimaze, Actiq, ... +5 more
Professional resources
Other brands
Duragesic, Sublimaze, Actiq, Fentora, ... +2 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.