Zejula
Generic name: niraparib [ nye-RAP-a-rib ]
Drug class: PARP inhibitors
What is Zejula?
Zejula is used as a "maintenance" treatment in adults to keep certain types of cancer from coming back. This includes cancers of the ovary (female reproductive organs where eggs are formed), fallopian tube (tube that transports eggs released by the ovaries to the uterus), or peritoneum (the membrane that lines the inside of your abdomen and covers some of your internal organs).
Zejula is given after you have received chemotherapy (with cisplatin, oxaliplatin, carboplatin, or other similar products) and your cancer has responded to that medicine.
Zejula is sometimes used only if your cancer has a specific genetic marker (an abnormal "BRCA" gene) or other gene mutations. Your doctor will make sure you have the correct tumor type to be treated with niraparib.
Zejula belongs to a class of medications called poly (ADP-ribose) polymerase (PARP) inhibitors. Niraparib works by interfering with the growth and spread of cancer cells in the body.
Zejula side effects
Get emergency medical help if you have signs of an allergic reaction to Zejula: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Stop taking this medicine and call your doctor at once if you have signs of a bone marrow disorder: fever, frequent infections, weakness, tiredness, feeling short of breath, weight loss, blood in your urine or stools, easy bruising or bleeding.
Zejula may cause serious side effects. Call your doctor at once if you have:
-
pounding heartbeats or fluttering in your chest;
-
sores or white patches in or around your mouth, trouble swallowing or talking, dry mouth, bad breath, altered sense of taste;
-
pain or burning when you urinate; or
-
headache, vision changes, confusion, seizure with our without pounding in your neck or ears.
Your cancer treatments may be delayed or permanently discontinued if you have certain side effects.
Common Zejula side effects may include:
-
abnormal blood tests;
-
little or no urination, changes in the color of your urine, painful urination;
-
back or muscle pain;
-
headache, dizziness;
-
sleep problems (insomnia);
-
tiredness;
-
cough, shortness of breath; or
-
rash.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Before taking this medicine
To make sure Zejula is safe for you, tell your doctor if you have ever had:
-
an allergy or sensitivity to aspirin or tartrazine (food dye);
You may need to have a negative pregnancy test before starting treatment with niraparib.
Niraparib may harm an unborn baby. Do not use if you are pregnant. Use effective birth control while using niraparib and for at least 6 months after your last dose. Tell your doctor if you become pregnant.
It may be harder for you to get a woman pregnant while you are using this medicine.
Do not breastfeed while using this medicine, and for at least 1 month after your last dose.
How should I take Zejula?
Take Zejula as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
You may take Zejula with or without food, but take it at the same time each day.
Take this medicine at bedtime if it upsets your stomach.
Swallow the tablet or capsule whole and do not crush, chew, break, or open it.
If you vomit shortly after taking Zejula, do not take another dose. Take your next dose as scheduled.
You may need frequent medical tests and your cancer treatments may be delayed based on the results.
Your blood pressure and heart rate will need to be checked often.
Store this medicine in the original container at room temperature away from moisture and heat.
Dosing Information
Usual Adult Dose for Ovarian Cancer:
300 mg orally once a day until disease progression or unacceptable toxicity
Usual Adult Dose for Fallopian Tube Cancer:
300 mg orally once a day until disease progression or unacceptable toxicity
Usual Adult Dose for Peritoneal Cancer:
300 mg orally once a day until disease progression or unacceptable toxicity
Comments:
-Initiate therapy with this drug no later than 8 weeks after a patient's most recent platinum-containing regimen.
Uses:
-For maintenance therapy of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy
-For treatment of adult patients with advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with 3 or more prior chemotherapy regimens and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either:
a deleterious or suspected deleterious BRCA mutation
OR
genomic instability and who have progressed more than 6 months after response to the last platinum-based chemotherapy
What happens if I miss a dose?
Skip the missed dose and use your next dose at the regular time. Do not use two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
What should I avoid while taking Zejula?
Follow your doctor's instructions about any restrictions on food, beverages, or activity.
Warnings
Stop taking Zejula and call your doctor at once if you have fever, frequent infections, weakness, tiredness, shortness of breath, weight loss, blood in your urine or stools, easy bruising or bleeding. These may be symptoms of bone marrow disorder and may lead to death.
You should not use Zejula if you are pregnant. Avoid pregnancy for at least 6 months after you stop using this medicine.
You should not breast-feed while using Zejula and for at least 1 month after your last dose.
What other drugs will affect Zejula?
Other drugs may interact with Zejula, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all other medicines you use.
How supplied
Zejula is available as oval-shaped, film-coated tablets containing 100 mg, 200 mg, or 300 mg of niraparib.
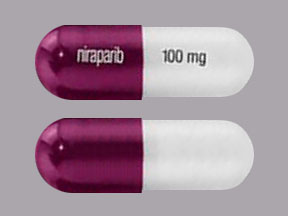
Zejula is also available in capsules containing 100 mg of niraparib. The capsules have a white body printed with “100 mg” in black ink, and a purple cap printed with “Niraparib” in white ink.
Ingredients
Active ingredient: niraparib. .
Inactive ingredients:
Capsule fill: magnesium stearate and lactose monohydrate.
Capsule shell: titanium dioxide and gelatin in the white capsule body and FD& C Blue No. 1, FD& C Red No. 3, FD& C Yellow No. 5 (tartrazine), and gelatin in the purple capsule cap. The black printing ink: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide, and black iron oxide. The white printing ink: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, sodium hydroxide, povidone, and titanium dioxide.
Core tablet: crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and silicon dioxide.
Tablet film-coating: Opadry II Gray (100 mg), Opadry II Blue (200 mg), or Opadry II Green (300 mg).
Manufacturer
GlaxoSmithKline, Durham, NC 27701, USA.
Frequently asked questions
References
More about Zejula (niraparib)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (56)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: PARP inhibitors
- Breastfeeding
- En español
Professional resources
Related treatment guides
Further information
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use Zejula only for the indication prescribed.
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Copyright 1996-2024 Cerner Multum, Inc. Version: 5.01.