Liquamycin LA-200 (Canada)
This page contains information on Liquamycin LA-200 for veterinary use.The information provided typically includes the following:
- Liquamycin LA-200 Indications
- Warnings and cautions for Liquamycin LA-200
- Direction and dosage information for Liquamycin LA-200
Liquamycin LA-200
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
Oxytetracycline Injection
Veterinary Use Only
A long-acting broad-spectrum antibiotic
DIN 02157209
Description
Liquamycin LA-200 is a unique formulation providing sustained antibiotic blood level action over a period of days following a single treatment. This is a sterile, clear, stable, ready-to-use injectable solution containing oxytetracycline (C22H24N2O9) as medicinal ingredient. The chemical structure of oxytetracycline is:
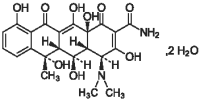
Each mL of Liquamycin LA-200 contains 200 mg of oxytetracycline (as oxytetracycline dihydrate) as medicinal ingredient and the following excipients: 400 mg of pyrrolidone, 50 mg of povidone, 17.2 mg of magnesium oxide, 2.4 mg of monoethanolamide, and 2.2 mg of sodium formaldehyde sulfoxylate.
Sustained Antibiotic Action:
Oxytetracycline has long been recognized as the proven drug of choice for the treatment of a wide variety of diseases caused by susceptible gram-positive and gram-negative bacteria. The use of parenterally-administered oxytetracycline in the management of diseases is based on an accurate diagnosis and an adequate course of treatment that normally consists of repeated injections over a three-day period.
There are many instances where a prolonged course of parenteral antibiotic therapy is desirable, but impractical due to the inaccessibility of the animal and extra labour costs associated with daily retreatment practices. Liquamycin LA- 200 injection is ideally suited for use in these instances.
The sustained action of Liquamycin LA-200 injection is believed to be in large part due to a controlled precipitation of oxytetracycline at the injection site. In effect, a depot of oxytetracycline is formed and the drug is absorbed and distributed throughout the body more slowly. Several factors are believed to play a role including: (1) the high concentration of Liquamycin LA-200 injection results in a proportionately smaller area for drug absorption from the site of injection than that of less concentrated formulations; (2) the inherent and unique composition of the formulation allows “limited” and controlled precipitation of the drug to occur. If precipitation was not controlled and too much of the drug precipitated at the injection site, blood levels would be markedly lower, accompanied by severe and long-lasting tissue damage at the injection site.
Liquamycin LA-200 injection provides broad-spectrum effectiveness and sustained antibiotic blood levels from a single intramuscular injection in cattle, sheep and swine, or from a single subcutaneous injection in cattle.
Liquamycin LA-200 Indications
Cattle: For the treatment of bacterial pneumonia, pasteurellosis (associated with the shipping fever complex) and footrot caused or complicated by bacteria susceptible to oxytetracycline, and for the treatment of pinkeye (infectious bovine keratoconjunctivitis) caused by Moraxella bovis.
Sheep: For the treatment of bacterial pneumonia in lambs caused by susceptible strains of Pasteurella multocida and Mannheimia haemolytica. For the treatment of ovine footrot.
Swine: For the treatment of bacterial pneumonia caused or complicated by bacteria susceptible to oxytetracycline.
Dosage and Administration
Liquamycin LA-200 injection is designed for intramuscular or subcutaneous administration in cattle, and intramuscular administration in sheep and swine at the single dose rate of 1 mL per 10 kg body weight, thus providing 20 mg oxytetracycline per kg body weight.
Animals should be restrained to ensure that treatment can be properly administered. Use sterile equipment and follow aseptic procedures. Each injection should be made using a clean, dry, 16 gauge needle.
Subcutaneous injections in cattle should be administered into the neck region, just in front of the shoulder, using a needle 1/2 to 5/8 inches in length. Intramuscular injections should be made deep into the fleshy part of the muscle, preferably in the lateral neck region, using a 1 1/2 inch needle.
Intramuscular injection in sheep should be administered using a 1 inch needle.
To minimize pain and local tissue irritation at the injection site in cattle and sheep it is recommended that the dose be divided and administered into separate injection sites; no more than 10 mL should be injected into any one site in cattle and no more than 5 mL into any one site in sheep.
For young pigs less than 10 kg body weight, it is recommended that they receive a dose of 1 mL per animal. For pigs up to 100 kg body weight, the dose may be injected into one site, but for pigs over 100 kg body weight the dose should be divided to minimize pain and local tissue irritation, and administered into two injection sites.
If clinical improvement is not observed within 48 to 72 hours following injection, the diagnosis should be re-evaluated.
Note:
In severe disease cases, treatment with Liquamycin LA-200 injection only, may be insufficient.
Warnings
Treated cattle, sheep and swine must not be slaughtered for use in food for at least 28 days after the latest intramuscular administration, or for at least 48 days in cattle after the latest subcutaneous administration of this drug. Do not use in lactating dairy cattle or lactating dairy ewes. Keep out of reach of children.
Cautions:
Occasional sensitivity reactions (anaphylaxis) to oxytetracycline have been known to occur. In such cases, administer epinephrine immediately.
Due to the high concentration and long acting effect, temporary swelling at the injection site may be observed for up to five days following injection.
Warm Liquamycin LA-200 injection to body temperature before administering.
Storage
Store between 15 and 30°C. Use within 28 days after first vial puncture. Puncture a maximum of 40 times, using a 18 gauge needle.
Presentation:
Liquamycin LA-200 injection is available in multi-dose 250 mL and 500 mL vials. The product is ready to use and requires no further preparation.
Not all pack sizes may be marketed.
Zoetis®, Liquamycin and LA-200 are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
7656-11-3
40040456
June 2023
CPN: 1198059.6
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
