Baytril Otic (Canada)
This page contains information on Baytril Otic for veterinary use.The information provided typically includes the following:
- Baytril Otic Indications
- Warnings and cautions for Baytril Otic
- Direction and dosage information for Baytril Otic
Baytril Otic
This treatment applies to the following species: Company: Elanco
Company: Elanco
(enrofloxacin/silver sulfadiazine)
Antibacterial-Antimycotic Emulsion
DIN 02246715
For Ototopical Use In Dogs
FOR VETERINARY USE ONLY
PRODUCT DESCRIPTION: Each milliliter of Baytril Otic contains: Medicinal ingredients; enrofloxacin 5 mg (0.5% w/v), silver sulfadiazine (SSD) 10 mg (1.0% w/v). Non-medicinal ingredients; benzyl alcohol (as a preservative) and cetylstearyl alcohol (as a stabilizer) in a neutral oil and purified water emulsion. The active ingredients are delivered via a physiological carrier (a non-irritating emulsion).
CHEMICAL NOMENCLATURE AND STRUCTURE:
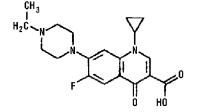
Enrofloxacin
1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1, 4-dihydro-4-oxo-3-quinolinecarboxylic acid.
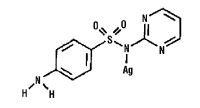
Silver Sulfadiazine
Benzenesulfonamide, 4-amino-N-2-pyrimidinyl-monosilver
ACTIONS: Enrofloxacin, a 4-fluoroquinolone compound, is bactericidal with activity against a broad spectrum of both Gram negative and Gram positive bacteria. Fluoroquinolones elicit their bactericidal activities through interactions with two intracellular enzymes, DNA gyrase (DNA topoisomerase II) and DNA topoisomerase IV, which are essential for bacterial DNA transcription, synthesis and replication. It is believed that fluoroquinolones actively bind with bacterial DNA:ENZYME complexes and thereby inhibit the essential processes catalyzed by the enzymes (DNA supercoiling and chromosomal decatenation).1 The ultimate outcome of the fluoroquinolone intervention is DNA fragmentation and bacterial cell death.2,3
Silver sulfadiazine (SSD) is synthesized from silver nitrate and sodium sulfadiazine.4 This compound has a wide spectrum of antimicrobial activity against Gram negative and Gram positive bacteria and is also an effective antimycotic.5,6 SSD suppresses microbial growth through inhibition of DNA replication and modification of the cell membrane.
MICROBIOLOGY: In clinical field trials, Baytril Otic demonstrated elimination or reduction of clinical signs associated with otitis externa and in vitro activity against cultured organisms. Baytril Otic is effective when used as a treatment for canine otitis externa associated with one or more of the following organisms: Malassezia pachydermatis, coagulase-positive Staphylococcus spp., Pseudomonas aeruginosa, Enterobacter spp., Proteus mirabilis, Streptococci spp., Aeromonas hydrophila, Aspergillus spp., Klebsiella pneumoniae, and Candida albicans.
In vitro assays, such as disk-diffusion and agar/broth-dilution, are used to determine the susceptibilities of microbes to antimicrobial therapies. Results of agar/broth-dilution assays are reported as a Minimal Inhibitory Concentration (MIC) which represents the lowest antimicrobial concentration, expressed in µg/mL, capable of inhibiting the growth of a pathogenic microorganism. MICs are used in conjunction with pharmacokinetics to predict the in vivo efficacy of systemically administered antimicrobials. Topical administration of Baytril Otic to an exudate and debris-free canal, however, will generally result in local antimicrobial concentrations that greatly exceed serum and tissue levels resulting from systemic therapy. Therefore, when using Baytril Otic as a treatment for canine otitis externa, interpret susceptibility data cautiously.
Baytril Otic Indications
Baytril Otic is indicated as a treatment for canine otitis externa complicated by bacterial and fungal organisms susceptible to enrofloxacin and/or silver sulfadiazine (see Microbiology section).
EFECTIVENESS: Due to its combination of active ingredients, Baytril Otic provides antimicrobial therapy against bacteria and fungi (which includes yeast) commonly encountered in cases of canine otitis externa.
The effectiveness of Baytril Otic was evaluated in a controlled, double-blind, multi-site clinical trial. One hundred and sixty-nine dogs (n=169), with naturally occurring active otitis externa participated in the study. The presence of active disease was verified by aural cytology, microbial culture and otoscopy/clinical scoring. Qualified cases were randomly assigned to either Baytril Otic treatment (n=113) or to a comparable placebo-based regimen (n=56). Treatments were administered twice daily for 7 or 14 days. Assessment of effectiveness was based on continued resolution of clinical signs 3 to 4 days following administration of the last dose.
At study conclusion, Baytril Otic was found to be a significantly more effective treatment for canine otitis externa than the placebo regimen. Based on the scoring system used to assess treatment response, therapeutic success occurred in 67% of Baytril Otic-treated infections compared to 14% with placebo. Approximately 75% of therapeutic successes were associated with the maximum treatment duration of 14 days.
Contraindications
Baytril Otic is contraindicated in dogs with suspected or known hypersensitivity to quinolones and/or sulfonamides.
Warning
To limit the potential development of antimicrobial resistance:- fluoroquinolone drugs such as Baytril Otic should not be used indiscriminately
- Baytril Otic should not be used in food producing animals
Keep out of the reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation develops or persists following ocular or dermal exposures. Individuals with a history of hypersensitivity to quinolone compounds or antibacterials should avoid handling this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
Baytril Otic Caution
The use of Baytril Otic in dogs with perforated tympanic membranes has not been evaluated. Therefore, the integrity of the tympanic membrane should be evaluated before administering this product. If hearing or vestibular dysfunction is noted during the course of treatment, discontinue use of Baytril Otic.
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures.
Quinolone-class drugs have been associated with cartilage erosions in weightbearing joints and other forms of arthropathy in immature animals of various species.
The safe use of Baytril Otic in dogs used for breeding purposes, during pregnancy, or in lactating bitches, has not been evaluated.
Adverse Reactions
During clinical trials, 2 of 113 (1.7%) dogs exhibited reactions that may have resulted from treatment with Baytril Otic. Both cases displayed local hypersensitivity responses of the aural epithelium to some component within the Baytril Otic formulation. The reactions were characterized by acute inflammation of the ear canal and pinna.To report suspected adverse drug events or for technical assistance, contact Elanco Canada Limited at 1-800-265-5475.
SAFETY: General Safety Study: In a target animal safety study, Baytril Otic was administered in both ears of 24 clinically normal beagle dogs at either recommended or exaggerated dosages: 10, 30 or 50 drops applied twice daily for 42 consecutive days. A control group of 8 beagle dogs was treated by administering 50 drops of vehicle in one ear twice daily for 42 consecutive days, with the contralateral ear untreated. Erythema was noted in all groups, including both treated and untreated ears in the controls, which resolved following termination of treatment.
Oral Safety Study: In order to test safety in case of ingestion, Baytril Otic was administered, twice daily for 14 consecutive days, to the dorsum of the tongue and to the left buccal mucosa of 6 clinically normal dogs. No adverse local or systemic reactions were reported.
Dosage and Administration
Exudate or debris within the ear canal may interfere with treatment and should be removed with a suitable non-irritating solution prior to administration of Baytril Otic.Shake well before each use.
Tilt head so that the affected ear is presented in an upward orientation. Administer a sufficient quantity of Baytril Otic to coat the aural lesions and the external auditory canal. As a general guide, administer 5-10 drops per ear treated in dogs weighing 16 kg or less and 10-15 drops per ear treated in dogs weighing more than 16 kg. Following treatment, gently massage the ear so as to ensure complete and uniform distribution of the medication throughout the external ear canal. Apply twice daily for a duration of up to 14 days.
Storage
Store between 4 and 25°C. Store in an upright position. Do not store in direct sunlight.How Supplied
Baytril Otic (enrofloxacin/silver sulfadiazine)|
Size |
Presentation |
|
15 mL |
Oval plastic bottle with dropper tip and extended tip closure |
References
1. Hooper DC and Wolfson JS. Mechanisms of quinolone action and bacterial killing, in Quinolone Antimicrobial Agents. Washington DC, American Society for Microbiology, 2nd ed., 1993, 53-75.
2. Gootz TD and Brightly KE. Fluoroquinolone antibacterial: mechanism of action, resistance and clinical aspects. Medicinal Research Reviews 1996; 16 (5): 433-486.
3. Drlica K and Zhoa X. DNA gyrase, topoisomerase IV and the 4-quinolones. Microbiology and Molecular Biology Reviews 1997; 61(3): 377-392.
4. Fox CL. Silver sulfadiazine: a new topical therapy for Pseudomonas in burns. Archives of Surgery 1968; 96:184-188.
5. Wlodkowski TJ and Rosenkranz HS. Antifungal activity of silver sulfadiazine. Lancet 1973; 2:739-740.
6. Schmidt A. In vitro activity of climbazole, clotrimazole and silver sulfadiazine against isolates of Malassezia pachydermatis. J of Vet Medicine Series B 1997; 44: 193-197.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401 Mississauga, Ontario L5N 0C9
Baytril is sold by Elanco or its affiliates and is not a product of Bayer. The Product Name Baytril is owned by Bayer and used under license.
© 2022 Elanco or its affiliates.
26Oct2022
CPN: 1231205.1
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
