Zostavax Prescribing Information
Package insert / product label
Generic name: zoster vaccine live
Dosage form: subcutaneous injection, powder, lyophilized, for suspension subcutaneous injection
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Jun 8, 2022.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ZOSTAVAX® (Zoster Vaccine Live)
Suspension for subcutaneous injection
Initial U.S. Approval: 2006
Indications and Usage for Zostavax
ZOSTAVAX is a live attenuated virus vaccine indicated for prevention of herpes zoster (shingles) in individuals 50 years of age and older. (1)
Limitations of Use of ZOSTAVAX:
Zostavax Dosage and Administration
Single 0.65 mL subcutaneous injection (2.1)
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- Hypersensitivity reactions including anaphylaxis have occurred with ZOSTAVAX (5.1)
- Transmission of vaccine virus may occur between vaccinees and susceptible contacts (5.2)
- Deferral should be considered in acute illness (for example, in the presence of fever) or in patients with active untreated tuberculosis (5.3)
- Avoid pregnancy for 3 months following vaccination with ZOSTAVAX (8.1)
Adverse Reactions/Side Effects
The most frequent adverse reactions, reported in ≥1% of subjects vaccinated with ZOSTAVAX, were headache and injection-site reactions. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Drug Interactions
In a randomized clinical study, a reduced immune response to ZOSTAVAX as measured by gpELISA was observed in individuals who received concurrent administration of PNEUMOVAX® 23 and ZOSTAVAX compared with individuals who received these vaccines 4 weeks apart. Consider administration of the two vaccines separated by at least 4 weeks. (7.1, 14.4)
Use In Specific Populations
Pregnancy: Do not administer ZOSTAVAX to females who are pregnant. Pregnancy should be avoided for 3 months following vaccination with ZOSTAVAX. (4.3, 8.1, 17)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2019
Related/similar drugs
Shingrix, zoster vaccine, recombinant
Full Prescribing Information
1. Indications and Usage for Zostavax
ZOSTAVAX® is a live attenuated virus vaccine indicated for prevention of herpes zoster (shingles) in individuals 50 years of age and older.
Limitations of Use of ZOSTAVAX:
- ZOSTAVAX is not indicated for the treatment of zoster or postherpetic neuralgia (PHN).
- ZOSTAVAX is not indicated for prevention of primary varicella infection (Chickenpox).
2. Zostavax Dosage and Administration
Subcutaneous administration only. Do not inject intravascularly or intramuscularly.
2.1 Recommended Dose and Schedule
Administer ZOSTAVAX as a single 0.65-mL dose subcutaneously in the deltoid region of the upper arm.
2.2 Preparation for Administration
Use only sterile syringes free of preservatives, antiseptics, and detergents for each injection and/or reconstitution of ZOSTAVAX. Preservatives, antiseptics and detergents may inactivate the vaccine virus.
ZOSTAVAX is stored frozen and should be reconstituted immediately upon removal from the freezer.
When reconstituted, ZOSTAVAX is a semi-hazy to translucent, off-white to pale yellow liquid.
Reconstitution:
- Use only the diluent supplied.
- Withdraw the entire contents of the diluent into a syringe.
- To avoid excessive foaming, slowly inject all of the diluent in the syringe into the vial of lyophilized vaccine and gently agitate to mix thoroughly.
- Withdraw the entire contents of reconstituted vaccine into a syringe, inject the total volume subcutaneously, and discard vial.
- ADMINISTER IMMEDIATELY AFTER RECONSTITUTION to minimize loss of potency. Discard reconstituted vaccine if not used within 30 minutes. Do not freeze reconstituted vaccine.
3. Dosage Forms and Strengths
ZOSTAVAX is a lyophilized preparation of live, attenuated varicella-zoster virus (Oka/Merck) to be reconstituted with sterile diluent to give a single dose suspension with a minimum of 19,400 PFU (plaque forming units) when stored at room temperature for up to 30 minutes.
4. Contraindications
4.1 Hypersensitivity
Do not administer ZOSTAVAX to individuals with a history of anaphylactic/anaphylactoid reaction to gelatin, neomycin or any other component of the vaccine. Neomycin allergy manifested as contact dermatitis is not a contraindication to receiving this vaccine. {1}
4.2 Immunosuppression
Do not administer ZOSTAVAX to individuals who are immunodeficient or immunosuppressed due to disease or therapy, as serious or fatal disseminated vaccine strain varicella-zoster virus disease may occur. Causes of immunodeficiency or immunosuppression may include, but are not limited to, primary or acquired immunodeficiency states, AIDS or other clinical manifestations of infection with human immunodeficiency viruses, leukemia, lymphoma or other malignant neoplasms affecting the bone marrow or lymphatic system, and immunosuppressive therapy.
4.3 Pregnancy
Do not administer ZOSTAVAX to pregnant women. Naturally occurring varicella-zoster virus (VZV) infection is known to sometimes cause fetal harm. Pregnancy should be avoided for 3 months following administration of ZOSTAVAX [see Use in Specific Populations (8.1) and Patient Counseling Information (17)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Serious adverse reactions, including anaphylaxis, have occurred with ZOSTAVAX. Adequate treatment provisions, including epinephrine injection (1:1,000), should be available for immediate use should an anaphylactic/anaphylactoid reaction occur.
5.2 Transmission of Vaccine Virus
Transmission of vaccine virus may occur between vaccinees and susceptible contacts.
6. Adverse Reactions/Side Effects
The most frequent adverse reactions, reported in ≥1% of subjects vaccinated with ZOSTAVAX, were headache and injection-site reactions.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, rates of adverse reactions observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
ZOSTAVAX Efficacy and Safety Trial (ZEST) in Subjects 50 to 59 Years of Age
In the ZEST study, subjects received a single dose of either ZOSTAVAX (N=11,184) or placebo (N=11,212). The racial distribution across both vaccination groups was similar: White (94.4%); Black (4.2%); Hispanic (3.3%) and Other (1.4%) in both vaccination groups. The gender distribution was 38% male and 62% female in both vaccination groups. The age distribution of subjects enrolled, 50 to 59 years, was similar in both vaccination groups. All subjects received a vaccination report card (VRC) to record adverse events occurring from Days 1 to 42 postvaccination.
In the ZEST study, serious adverse events occurred at a similar rate in subjects vaccinated with ZOSTAVAX (0.6%) or placebo (0.5%) from Days 1 to 42 postvaccination.
In the ZEST study, all subjects were monitored for adverse reactions. An anaphylactic reaction was reported for one subject vaccinated with ZOSTAVAX.
Most Common Adverse Reactions and Experiences in the ZEST Study
The overall incidence of vaccine-related injection-site adverse reactions within 5 days postvaccination was greater for subjects vaccinated with ZOSTAVAX as compared to subjects who received placebo (63.6% for ZOSTAVAX and 14.0% for placebo). Injection-site adverse reactions occurring at an incidence ≥1% within 5 days postvaccination are shown in Table 1.
|
||
|
Injection-Site Adverse Reaction |
ZOSTAVAX (N = 11094) % |
Placebo (N = 11116) % |
|
Solicited* Pain Erythema Swelling |
53.9 48.1 40.4 |
9.0 4.3 2.8 |
|
Unsolicited Pruritus Warmth Hematoma Induration |
11.3 3.7 1.6 1.1 |
0.7 0.2 1.6 0.0 |
Systemic adverse reactions and experiences reported during Days 1-42 at an incidence of ≥1% in either vaccination group were headache (ZOSTAVAX 9.4%, placebo 8.2%) and pain in the extremity (ZOSTAVAX 1.3%, placebo 0.8%), respectively.
The overall incidence of systemic adverse experiences reported during Days 1-42 was higher for ZOSTAVAX (35.4%) than for placebo (33.5%).
Shingles Prevention Study (SPS) in Subjects 60 Years of Age and Older
In the SPS, the largest clinical trial of ZOSTAVAX, subjects received a single dose of either ZOSTAVAX (n=19,270) or placebo (n=19,276). The racial distribution across both vaccination groups was similar: White (95%); Black (2.0%); Hispanic (1.0%) and Other (1.0%) in both vaccination groups. The gender distribution was 59% male and 41% female in both vaccination groups. The age distribution of subjects enrolled, 59-99 years, was similar in both vaccination groups.
The Adverse Event Monitoring Substudy of the SPS, designed to provide detailed data on the safety profile of the zoster vaccine (n=3,345 received ZOSTAVAX and n=3,271 received placebo) used vaccination report cards (VRC) to record adverse events occurring from Days 0 to 42 postvaccination (97% of subjects completed VRC in both vaccination groups). In addition, monthly surveillance for hospitalization was conducted through the end of the study, 2 to 5 years postvaccination.
The remainder of subjects in the SPS (n=15,925 received ZOSTAVAX and n=16,005 received placebo) were actively followed for safety outcomes through Day 42 postvaccination and passively followed for safety after Day 42.
Serious Adverse Events Occurring 0-42 Days Postvaccination
In the overall SPS study population, serious adverse events occurred at a similar rate (1.4%) in subjects vaccinated with ZOSTAVAX or placebo.
In the AE Monitoring Substudy, the rate of SAEs was increased in the group of subjects who received ZOSTAVAX as compared to the group of subjects who received placebo (Table 2).
| Cohort | ZOSTAVAX n/N % | Placebo n/N % | Relative Risk (95% CI) |
|---|---|---|---|
| N=number of subjects in cohort with safety follow-up n=number of subjects reporting an SAE 0-42 Days postvaccination |
|||
| Overall Study Cohort (60 years of age and older) | 255/18671 1.4% | 254/18717 1.4% | 1.01 (0.85, 1.20) |
| 60-69 years old | 113/10100 1.1% | 101/10095 1.0% | 1.12 (0.86, 1.46) |
| 70-79 years old | 115/7351 1.6% | 132/7333 1.8% | 0.87 (0.68, 1.11) |
| ≥80 years old | 27/1220 2.2% | 21/1289 1.6% | 1.36 (0.78, 2.37) |
| AE Monitoring Substudy Cohort (60 years of age and older) | 64/3326 1.9% | 41/3249 1.3% | 1.53 (1.04, 2.25) |
| 60-69 years old | 22/1726 1.3% | 18/1709 1.1% | 1.21 (0.66, 2.23) |
| 70-79 years old | 31/1383 2.2% | 19/1367 1.4% | 1.61 (0.92, 2.82) |
| ≥80 years old | 11/217 5.1% | 4/173 2.3% | 2.19 (0.75, 6.45) |
Among reported serious adverse events in the SPS (Days 0 to 42 postvaccination), serious cardiovascular events occurred more frequently in subjects who received ZOSTAVAX (20 [0.6%]) than in subjects who received placebo (12 [0.4%]) in the AE Monitoring Substudy. The frequencies of serious cardiovascular events were similar in subjects who received ZOSTAVAX (81 [0.4%]) and in subjects who received placebo (72 [0.4%]) in the entire study cohort (Days 0 to 42 postvaccination).
Serious Adverse Events Occurring Over the Entire Course of the Study
Rates of hospitalization were similar among subjects who received ZOSTAVAX and subjects who received placebo in the AE Monitoring Substudy, throughout the entire study.
Fifty-one individuals (1.5%) receiving ZOSTAVAX were reported to have congestive heart failure (CHF) or pulmonary edema compared to 39 individuals (1.2%) receiving placebo in the AE Monitoring Substudy; 58 individuals (0.3%) receiving ZOSTAVAX were reported to have congestive heart failure (CHF) or pulmonary edema compared to 45 (0.2%) individuals receiving placebo in the overall study.
In the SPS, all subjects were monitored for vaccine-related SAEs. Investigator-determined, vaccine-related serious adverse experiences were reported for 2 subjects vaccinated with ZOSTAVAX (asthma exacerbation and polymyalgia rheumatica) and 3 subjects who received placebo (Goodpasture's syndrome, anaphylactic reaction, and polymyalgia rheumatica).
Deaths
The incidence of death was similar in the groups receiving ZOSTAVAX or placebo during the Days 0-42 postvaccination period; 14 deaths occurred in the group of subjects who received ZOSTAVAX and 16 deaths occurred in the group of subjects who received placebo. The most common reported cause of death was cardiovascular disease (10 in the group of subjects who received ZOSTAVAX, 8 in the group of subjects who received placebo). The overall incidence of death occurring at any time during the study was similar between vaccination groups: 793 deaths (4.1%) occurred in subjects who received ZOSTAVAX and 795 deaths (4.1%) in subjects who received placebo.
Most Common Adverse Reactions and Experiences in the AE Monitoring Substudy of the SPS
Injection-site adverse reactions reported at an incidence ≥1% are shown in Table 3. Most of these adverse reactions were reported as mild in intensity. The overall incidence of vaccine-related injection-site adverse reactions was significantly greater for subjects vaccinated with ZOSTAVAX versus subjects who received placebo (48% for ZOSTAVAX and 17% for placebo).
| Adverse Reaction |
ZOSTAVAX (N = 3345) % |
Placebo (N = 3271) % |
|
Solicited† Erythema Pain/Tenderness Swelling |
35.6 34.3 26.1 |
6.9 8.3 4.5 |
|
Unsolicited Hematoma Pruritus Warmth |
1.6 6.9 1.6 |
1.4 1.0 0.3 |
Headache was the only systemic adverse reaction reported on the vaccine report card between Days 0-42 by ≥1% of subjects in the AE Monitoring Substudy in either vaccination group (ZOSTAVAX 1.4%, placebo 0.8%).
The numbers of subjects with elevated temperature (≥38.3°C [≥101.0°F]) within 42 days postvaccination were similar in the ZOSTAVAX and the placebo vaccination groups [27 (0.8%) vs. 27 (0.9%), respectively].
The following adverse experiences in the AE Monitoring Substudy of the SPS (Days 0 to 42 postvaccination) were reported at an incidence ≥1% and greater in subjects who received ZOSTAVAX than in subjects who received placebo, respectively: respiratory infection (65 [1.9%] vs. 55 [1.7%]), fever (59 [1.8%] vs. 53 [1.6%]), flu syndrome (57 [1.7%] vs. 52 [1.6%]), diarrhea (51 [1.5%] vs. 41 [1.3%]), rhinitis (46 [1.4%] vs. 36 [1.1%]), skin disorder (35 [1.1%] vs. 31 [1.0%]), respiratory disorder (35 [1.1%] vs. 27 [0.8%]), asthenia (32 [1.0%] vs. 14 [0.4%]).
6.2 VZV Rashes Following Vaccination
Within the 42-day postvaccination reporting period in the ZEST, noninjection-site zoster-like rashes were reported by 34 subjects (19 for ZOSTAVAX and 15 for placebo). Of 24 specimens that were adequate for Polymerase Chain Reaction (PCR) testing, wild-type VZV was detected in 10 (3 for ZOSTAVAX, 7 for placebo) of these specimens. The Oka/Merck strain of VZV was not detected from any of these specimens. Of reported varicella-like rashes (n=124, 69 for ZOSTAVAX and 55 for placebo), 23 had specimens that were available and adequate for PCR testing. VZV was detected in one of these specimens in the ZOSTAVAX group; however, the virus strain (wild-type or Oka/Merck strain) could not be determined.
Within the 42-day postvaccination reporting period in the SPS, noninjection-site zoster-like rashes were reported by 53 subjects (17 for ZOSTAVAX and 36 for placebo). Of 41 specimens that were adequate for Polymerase Chain Reaction (PCR) testing, wild-type VZV was detected in 25 (5 for ZOSTAVAX, 20 for placebo) of these specimens. The Oka/Merck strain of VZV was not detected from any of these specimens.
Of reported varicella-like rashes (n=59), 10 had specimens that were available and adequate for PCR testing. VZV was not detected in any of these specimens.
In clinical trials in support of the initial licensure of the frozen formulation of ZOSTAVAX, the reported rates of noninjection-site zoster-like and varicella-like rashes within 42 days postvaccination were also low in both zoster vaccine and placebo recipients. Of 17 reported varicella-like rashes and non-injection site zoster-like rashes, 10 specimens were available and adequate for PCR testing, and 2 subjects had varicella (onset Day 8 and 17) confirmed to be Oka/Merck strain.
6.3 Post-Marketing Experience
The following additional adverse reactions have been identified during post-marketing use of ZOSTAVAX. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
Gastrointestinal disorders: nausea
Infections and infestations: vaccine strain herpes zoster; disseminated vaccine strain varicella-zoster virus disease including fatal outcomes in immunocompromised patients
Skin and subcutaneous tissue disorders: rash
Musculoskeletal and connective tissue disorders: arthralgia; myalgia
General disorders and administration site conditions: injection-site rash; pyrexia; injection-site urticaria; transient injection-site lymphadenopathy
Immune system disorders: hypersensitivity reactions including anaphylactic reactions
Eye Disorders: necrotizing retinitis (patients on immunosuppressive therapy)
Nervous system disorders: Guillain-Barré syndrome; facial paralysis
Reporting Adverse Events
The U.S. Department of Health and Human Services has established a Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine. For information or a copy of the vaccine reporting form, call the VAERS toll-free number at 1-800-822-7967 or report online to www.vaers.hhs.gov. {2}
7. Drug Interactions
7.1 Concomitant Administration with Other Vaccines
In a randomized clinical study, a reduced immune response to ZOSTAVAX as measured by gpELISA was observed in individuals who received concurrent administration of PNEUMOVAX® 23 and ZOSTAVAX compared with individuals who received these vaccines 4 weeks apart. Consider administration of the two vaccines separated by at least 4 weeks [see Clinical Studies (14.4)].
For concomitant administration of ZOSTAVAX with inactivated influenza vaccine [see Clinical Studies (14.4)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
ZOSTAVAX is contraindicated for use in pregnant women because the vaccine contains live, attenuated varicella-zoster virus, and it is known that wild-type varicella-zoster virus, if acquired during pregnancy, can cause congenital varicella syndrome [see Contraindications (4.3) and Patient Counseling Information (17)].
Available data on inadvertent administration of ZOSTAVAX to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
It is not known whether varicella-zoster vaccine virus is excreted in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ZOSTAVAX, and any potential adverse effects on the breastfed child from ZOSTAVAX or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
11. Zostavax Description
ZOSTAVAX is a lyophilized preparation of the Oka/Merck strain of live, attenuated varicella-zoster virus (VZV). ZOSTAVAX, when reconstituted as directed, is a sterile suspension for subcutaneous administration. Each 0.65-mL dose contains a minimum of 19,400 PFU (plaque-forming units) of Oka/Merck strain of VZV when reconstituted and stored at room temperature for up to 30 minutes.
Each dose contains 31.16 mg of sucrose, 15.58 mg of hydrolyzed porcine gelatin, 3.99 mg of sodium chloride, 0.62 mg of monosodium L-glutamate, 0.57 mg of sodium phosphate dibasic, 0.10 mg of potassium phosphate monobasic, 0.10 mg of potassium chloride; residual components of MRC-5 cells including DNA and protein; and trace quantities of neomycin and bovine calf serum. The product contains no preservatives.
12. Zostavax - Clinical Pharmacology
12.1 Mechanism of Action
The risk of developing zoster appears to be related to a decline in VZV-specific immunity. ZOSTAVAX was shown to boost VZV-specific immunity, which is thought to be the mechanism by which it protects against zoster and its complications. [See Clinical Studies (14).]
Herpes zoster (HZ), commonly known as shingles or zoster, is a manifestation of the reactivation of varicella zoster virus (VZV), which, as a primary infection, produces chickenpox (varicella). Following initial infection, the virus remains latent in the dorsal root or cranial sensory ganglia until it reactivates, producing zoster. Zoster is characterized by a unilateral, painful, vesicular cutaneous eruption with a dermatomal distribution.
Pain associated with zoster may occur during the prodrome, the acute eruptive phase, and the postherpetic phase of the infection. Pain occurring in the postherpetic phase of infection is commonly referred to as postherpetic neuralgia (PHN).
Serious complications, such as PHN, scarring, bacterial superinfection, allodynia, cranial and motor neuron palsies, pneumonia, encephalitis, visual impairment, hearing loss, and death can occur as the result of zoster.
14. Clinical Studies
In two large clinical trials (ZEST and SPS), ZOSTAVAX significantly reduced the risk of developing zoster when compared with placebo (see Table 4 and Table 5).
14.1 ZOSTAVAX Efficacy and Safety Trial (ZEST) in Subjects 50 to 59 Years of Age
Efficacy of ZOSTAVAX was evaluated in the ZOSTAVAX Efficacy and Safety Trial (ZEST), a placebo-controlled, double-blind clinical trial in which 22,439 subjects 50 to 59 years of age were randomized to receive a single dose of either ZOSTAVAX (n=11,211) or placebo (n=11,228). Subjects were followed for the development of zoster for a median of 1.3 years (range 0 to 2 years). Confirmed zoster cases were determined by Polymerase Chain Reaction (PCR) [86%] or, in the absence of virus detection, by a Clinical Evaluation Committee [14%]. The primary efficacy analysis included all subjects randomized in the study (intent-to-treat [ITT] analysis).
Compared with placebo, ZOSTAVAX significantly reduced the risk of developing zoster by 69.8% (95% CI: 54.1, 80.6%) in subjects 50 to 59 years of age (Table 4).
|
|||||||
| ZOSTAVAX | Placebo | ||||||
| Age group (yrs.) | # subjects | # HZ cases | Incidence rate of HZ per 1000 person-yrs. | # subjects | # HZ cases | Incidence rate of HZ per 1000 person-yrs. | Vaccine Efficacy (95% CI) |
| 50-59 | 11211 | 30 | 1.994 | 11228 | 99 | 6.596 | 69.8% (54.1%, 80.6%) |
Immune responses to vaccination were evaluated in a random 10% subcohort (n=1,136 for ZOSTAVAX and n=1,133 for placebo) of the subjects enrolled in the ZEST study. VZV antibody levels (Geometric Mean Titers, GMT), as measured by glycoprotein enzyme-linked immunosorbent assay (gpELISA) 6 weeks postvaccination, were increased 2.3-fold (95% CI: 2.2, 2.4) in the group of subjects who received ZOSTAVAX compared to subjects who received placebo; the specific antibody level that correlates with protection from zoster has not been established.
14.2 Shingles Prevention Study (SPS) in Subjects 60 Years of Age and Older
Efficacy of ZOSTAVAX was evaluated in the Shingles Prevention Study (SPS), a placebo-controlled, double-blind clinical trial in which 38,546 subjects 60 years of age or older were randomized to receive a single dose of either ZOSTAVAX (n=19,270) or placebo (n=19,276). Subjects were followed for the development of zoster for a median of 3.1 years (range 31 days to 4.90 years). The study excluded people who were immunocompromised or using corticosteroids on a regular basis, anyone with a previous history of HZ, and those with conditions that might interfere with study evaluations, including people with cognitive impairment, severe hearing loss, those who were non-ambulatory, and those whose survival was not considered to be at least 5 years. Randomization was stratified by age, 60-69 and ≥70 years of age. Suspected zoster cases were confirmed by Polymerase Chain Reaction (PCR) [93%], viral culture [1%], or in the absence of virus detection, as determined by a Clinical Evaluation Committee [6%]. Individuals in both vaccination groups who developed zoster were given famciclovir, and, as necessary, pain medications. The primary efficacy analysis included all subjects randomized in the study who were followed for at least 30 days postvaccination and did not develop an evaluable case of HZ within the first 30 days postvaccination (Modified Intent-To-Treat [MITT] analysis).
ZOSTAVAX significantly reduced the risk of developing zoster when compared with placebo (Table 5). In the SPS, vaccine efficacy for the prevention of HZ was highest for those subjects 60-69 years of age and declined with increasing age.
| Age group† (yrs.) | ZOSTAVAX | Placebo |
Vaccine Efficacy (95% CI) |
||||
| # subjects | # HZ cases | Incidence rate of HZ per 1000 person-yrs. | # subjects | # HZ cases | Incidence rate of HZ per 1000 person-yrs. | ||
|
|||||||
| Overall | 19254 | 315 | 5.4 | 19247 | 642 | 11.1 | 51% (44%, 58%) |
| 60-69 | 10370 | 122 | 3.9 | 10356 | 334 | 10.8 | 64% (56%, 71%) |
| 70-79 | 7621 | 156 | 6.7 | 7559 | 261 | 11.4 | 41% (28%, 52%) |
| ≥80 | 1263 | 37 | 9.9 | 1332 | 47 | 12.2 | 18% (-29%, 48%) |
Forty-five subjects were excluded from the MITT analysis (16 in the group of subjects who received ZOSTAVAX and 29 in the group of subjects who received placebo), including 24 subjects with evaluable HZ cases that occurred in the first 30 days postvaccination (6 evaluable HZ cases in the group of subjects who received ZOSTAVAX and 18 evaluable HZ cases in the group of subjects who received placebo).
Suspected HZ cases were followed prospectively for the development of HZ-related complications. Table 6 compares the rates of PHN defined as HZ-associated pain (rated as 3 or greater on a 10-point scale by the study subject and occurring or persisting at least 90 days) following the onset of rash in evaluable cases of HZ.
|
Age group (yrs.)‡ | ZOSTAVAX | Placebo |
Vaccine efficacy against PHN in subjects who develop HZ postvaccination (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # subjects | # HZ cases | # PHN cases | Incidence rate of PHN per 1,000 person-yrs. | % HZ cases with PHN | # subjects | # HZ cases | # PHN cases | Incidence rate of PHN per 1,000 person-yrs. | % HZ cases with PHN | ||
|
|||||||||||
| Overall | 19254 | 315 | 27 | 0.5 | 8.6% | 19247 | 642 | 80 | 1.4 | 12.5% | 39%§
(7%, 59%) |
| 60-69 | 10370 | 122 | 8 | 0.3 | 6.6% | 10356 | 334 | 23 | 0.7 | 6.9% | 5% (-107%, 56%) |
| 70-79 | 7621 | 156 | 12 | 0.5 | 7.7% | 7559 | 261 | 45 | 2.0 | 17.2% | 55% (18%, 76%) |
| ≥80 | 1263 | 37 | 7 | 1.9 | 18.9% | 1332 | 47 | 12 | 3.1 | 25.5% | 26% (-69%, 68%) |
The median duration of clinically significant pain (defined as ≥3 on a 0-10 point scale) among HZ cases in the group of subjects who received ZOSTAVAX as compared to the group of subjects who received placebo was 20 days vs. 22 days based on the confirmed HZ cases.
Overall, the benefit of ZOSTAVAX in the prevention of PHN can be primarily attributed to the effect of the vaccine on the prevention of herpes zoster. Vaccination with ZOSTAVAX in the SPS reduced the incidence of PHN in individuals 70 years of age and older who developed zoster postvaccination. Other prespecified zoster-related complications were reported less frequently in subjects who received ZOSTAVAX compared to subjects who received placebo. Among HZ cases, zoster-related complications were reported at similar rates in both vaccination groups (Table 7).
| Complication | ZOSTAVAX (N = 19270) | Placebo (N = 19276) |
||
|---|---|---|---|---|
| (n = 321) | % Among Zoster Cases | (n = 659) | % Among Zoster Cases |
|
| N=number of subjects randomized n=number of zoster cases, including those cases occurring within 30 days postvaccination, with these data available |
||||
|
||||
| Allodynia | 135 | 42.1 | 310 | 47.0 |
| Bacterial Superinfection | 3 | 0.9 | 7 | 1.1 |
| Dissemination | 5 | 1.6 | 11 | 1.7 |
| Impaired Vision | 2 | 0.6 | 9 | 1.4 |
| Ophthalmic Zoster | 35 | 10.9 | 69 | 10.5 |
| Peripheral Nerve Palsies (motor) | 5 | 1.6 | 12 | 1.8 |
| Ptosis | 2 | 0.6 | 9 | 1.4 |
| Scarring | 24 | 7.5 | 57 | 8.6 |
| Sensory Loss | 7 | 2.2 | 12 | 1.8 |
Visceral complications reported by fewer than 1% of subjects with zoster included 3 cases of pneumonitis and 1 case of hepatitis in the placebo group, and 1 case of meningoencephalitis in the vaccine group.
Immune responses to vaccination were evaluated in a subset of subjects enrolled in the Shingles Prevention Study (N=1,395). VZV antibody levels (Geometric Mean Titers, GMT), as measured by glycoprotein enzyme-linked immunosorbent assay (gpELISA) 6 weeks postvaccination, were increased 1.7-fold (95% CI: 1.6 to 1.8) in the group of subjects who received ZOSTAVAX compared to subjects who received placebo; the specific antibody level that correlates with protection from zoster has not been established.
14.3 Long-Term Effectiveness Study in Individuals 50 Years of Age or Older
An interim analysis of a prospective observational cohort study conducted in a US integrated healthcare system database estimated vaccine effectiveness against HZ and PHN among 1,355,720 individuals 50 years of age and older, including 392,677 who received ZOSTAVAX. Age eligible individuals contributed person-time to the unvaccinated group and, once vaccinated with ZOSTAVAX, contributed person-time to the vaccinated group for the remainder of the study. Vaccine effectiveness (VE) against HZ and PHN was calculated using the incidence rates of protocol-defined first episode of HZ and PHN in the vaccinated and unvaccinated groups, including adjustments for calendar time, age, sex, race/ethnicity, healthcare resource utilization, comorbid conditions, and immunocompromise status. The gender and racial/ethnic distributions of study individuals were overall 53% female, 60% white, 15% Asian or Pacific Islander, 13% Hispanic, and 7% black or African American.
For individuals 50-59 years of age at the time of vaccination, the average VE against HZ over the first 3 years following vaccination was 60% (95% CI: 52, 66), with VE against HZ of 36% (95% CI: -55, 73) in the third year post-vaccination. For individuals 60-69 years of age, 70-79 years of age, and 80 years of age and older at the time of vaccination, the average VE against HZ over the first 5 years following vaccination was 49% (95% CI: 47, 52), 46% (95% CI: 43, 48), and 44% (95% CI: 38, 49), respectively, with VE against HZ of 34% (95% CI: 25, 42), 29% (95% CI: 18, 38), and 36% (95% CI: 12, 53), respectively, in the fifth year post-vaccination. Follow-up time for individuals 50-59 years of age was shorter because ZOSTAVAX was approved for use in this age group five years after approval for use in individuals 60 years of age and older.
Insufficient data were available at the time of the interim analysis to assess the outcome of PHN in individuals 50-59 years of age. For individuals 60-69 years of age, 70-79 years of age, and 80 years of age and older at the time of vaccination, the average VE against PHN over the first 5 years following vaccination was 72% (95% CI: 65, 77), 69% (95% CI: 62, 75), and 61% (95% CI: 47, 71), respectively, with VE against PHN of 61% (95% CI: 33, 77), 69% (95% CI: 44, 82), and 34% (95% CI: -49, 71), respectively, in the fifth year post-vaccination. The benefit of ZOSTAVAX in the prevention of PHN can be attributed in part to the effect of the vaccine on the prevention of HZ.
14.4 Concomitant Use Studies
In a double-blind, controlled substudy, 374 adults in the US, 60 years of age and older (median age = 66 years), were randomized to receive trivalent inactivated influenza vaccine (TIV) and ZOSTAVAX concurrently (N=188), or TIV alone followed 4 weeks later by ZOSTAVAX alone (N=186). The antibody responses to both vaccines at 4 weeks postvaccination were similar in both groups.
In another double-blind, controlled study, 882 adults in the US, 50 years of age and older (median age = 60 years), were randomized to receive quadrivalent inactivated influenza vaccine and ZOSTAVAX concurrently (N=440), or quadrivalent inactivated influenza vaccine alone followed 4 weeks later by ZOSTAVAX alone (N=442). The antibody responses to both vaccines at 4 weeks postvaccination were similar in both groups.
In a double-blind, controlled clinical trial, 473 adults, 60 years of age or older, were randomized to receive ZOSTAVAX and PNEUMOVAX 23 concomitantly (N=237), or PNEUMOVAX 23 alone followed 4 weeks later by ZOSTAVAX alone (N=236). At 4 weeks postvaccination, the VZV antibody levels following concomitant use were significantly lower than the VZV antibody levels following nonconcomitant administration (GMTs of 338 vs. 484 gpELISA units/mL, respectively; GMT ratio = 0.70 (95% CI: 0.61, 0.80).
15. References
- Reitschel RL, Bernier R. Neomycin sensitivity and the MMR vaccine. JAMA 1981;245(6):571.
- Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR 2002;51(RR02):1-36.
- Rynn L, Cragan J, Correa A. Update on Overall Prevalence of Major Birth Defects-Atlanta, Georgia,1978-2005. CDC MMWR January 11, 2008/57(01);1-5.
- American College of Obstetricians and Gynecologists Frequently Asked Questions: Miscarriage and Molar Pregnancy; 2011.
- Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: Adaptation of the brief pain inventory. J Pain 2004;5(6):344-56.
16. How is Zostavax supplied
No. 4963-00 — ZOSTAVAX is supplied as follows: (1) a package of 1 single-dose vial of lyophilized vaccine, NDC 0006-4963-00 (package A); and (2) a separate package of 10 vials of diluent (package B).
No. 4963-41 — ZOSTAVAX is supplied as follows: (1) a package of 10 single-dose vials of lyophilized vaccine, NDC 0006-4963-41 (package A); and (2) a separate package of 10 vials of diluent (package B).
Storage
To maintain potency, ZOSTAVAX must be stored frozen between -58°F and +5°F (-50°C and -15°C). Use of dry ice may subject ZOSTAVAX to temperatures colder than -58°F (-50°C).
Before reconstitution, ZOSTAVAX SHOULD BE STORED FROZEN at a temperature between -58°F and +5°F (-50°C and -15°C) until it is reconstituted for injection. Any freezer, including frost-free, that has a separate sealed freezer door and reliably maintains an average temperature between -58°F and +5°F (-50°C and -15°C) is acceptable for storing ZOSTAVAX. Routine defrost cycling of a frost-free freezer is acceptable.
ZOSTAVAX may be stored and/or transported at refrigerator temperature between 36°F and 46°F (2°C to 8°C) for up to 72 continuous hours prior to reconstitution. Vaccine stored between 36°F and 46°F (2°C to 8°C) that is not used within 72 hours of removal from +5°F (-15°C) storage should be discarded. ZOSTAVAX should be reconstituted immediately upon removal from the freezer. The diluent should be stored separately at room temperature (68°F to 77°F, 20°C to 25°C), or in the refrigerator (36°F to 46°F, 2°C to 8°C).
For information regarding the product or questions regarding storage conditions, call 1-800-MERCK-90.
Before reconstitution, protect from light.
DO NOT FREEZE RECONSTITUTED VACCINE.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Question the patient about reactions to previous vaccines.
- Provide a copy of the patient information (PPI) located at the end of this insert and discuss any questions or concerns.
- Inform patient of the benefits and risks of ZOSTAVAX, including the potential risk of transmitting the vaccine virus to susceptible individuals, such as immunosuppressed or immunodeficient individuals or pregnant women who have not had chickenpox.
- Instruct patient to report any adverse reactions or any symptoms of concern to their healthcare professional.
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
uspi-v211-i-fro-1909r029
Patient Information about
ZOSTAVAX® (pronounced "ZOS tah vax")
Generic name: Zoster Vaccine Live
You should read this summary of information about ZOSTAVAX before you are vaccinated. If you have any questions about ZOSTAVAX after reading this leaflet, you should ask your health care provider. This information does not take the place of talking about ZOSTAVAX with your doctor, nurse, or other health care provider. Only your health care provider can decide if ZOSTAVAX is right for you.
What is ZOSTAVAX and how does it work?
ZOSTAVAX is a vaccine that is used for adults 50 years of age or older to prevent shingles (also known as zoster).
ZOSTAVAX contains a weakened chickenpox virus (varicella-zoster virus).
ZOSTAVAX works by helping your immune system protect you from getting shingles.
If you do get shingles even though you have been vaccinated, ZOSTAVAX may help prevent the nerve pain that can follow shingles in some people. ZOSTAVAX does not protect everyone, so some people who get the vaccine may still get shingles.
ZOSTAVAX cannot be used to treat shingles, or the nerve pain that may follow shingles, once you have it.
What do I need to know about shingles and the virus that causes it?
Shingles is caused by the same virus that causes chickenpox. Once you have had chickenpox, the virus can stay in your nervous system for many years. For reasons that are not fully understood, the virus may become active again and give you shingles. Age and problems with the immune system may increase your chances of getting shingles.
Shingles is a rash that is usually on one side of the body. The rash begins as a cluster of small red spots that often blister. The rash can be painful. Shingles rashes usually last up to 30 days and, for most people, the pain associated with the rash lessens as it heals.
Who should not get ZOSTAVAX?
You should not get ZOSTAVAX if you:
- are allergic to any of its ingredients.
- are allergic to gelatin or neomycin.
- have a weakened immune system (for example, an immune deficiency, leukemia, lymphoma, or HIV/AIDS).
- take medicines or receive treatment that might weaken your immune system (such as high doses of steroids by injection or by mouth).
- are pregnant or plan to get pregnant.
You should not get ZOSTAVAX to prevent chickenpox.
Children should not get ZOSTAVAX.
How is ZOSTAVAX given?
ZOSTAVAX is given as a single dose by injection under the skin.
What should I tell my health care provider before I get ZOSTAVAX?
You should tell your health care provider if you:
- have or have had any medical problems.
- take or have taken any medicines, including non-prescription medicines, and dietary supplements.
- have any allergies, including allergies to neomycin or gelatin.
- had an allergic reaction to another vaccine.
- are pregnant or plan to become pregnant.
- are breast-feeding.
Tell your health care provider if you expect to be in close contact (including household contact) with newborn infants, someone who may be pregnant and has not had chickenpox or been vaccinated against chickenpox, or someone who has problems with their immune system. Your health care provider can tell you what situations you may need to avoid.
Can I get ZOSTAVAX with other vaccines?
ZOSTAVAX can be given at the same time as inactivated flu vaccine.
Talk to your health care provider if you plan to get ZOSTAVAX at the same time as PNEUMOVAX® 23 because it may be better to get these vaccines at least 4 weeks apart.
What are the possible side effects of ZOSTAVAX?
The most common side effects that people in the clinical studies reported after receiving the vaccine include:
- redness, pain, itching, swelling, hard lump, warmth, or bruising where the shot was given.
- headache
The following additional side effects have been reported with ZOSTAVAX:
- allergic reactions, which may be serious and may include difficulty in breathing or swallowing. If you have an allergic reaction, call your doctor right away.
- chickenpox
- fever
- hives at the injection site
- joint pain
- muscle pain
- nausea
- rash
- rash at the injection site
- shingles
- swollen glands near the injection site (that may last a few days to a few weeks)
- Guillain-Barré syndrome (muscle weakness, abnormal sensations, tingling in the arms, legs, and upper body)
- loss of facial muscle movements
Tell your healthcare provider if you have any new or unusual symptoms after you receive ZOSTAVAX. For a complete list of side effects, ask your health care provider.
Report the following to your doctor or your child's doctor:
- any adverse reactions following vaccination
- exposure to ZOSTAVAX during pregnancy
- exposure to ZOSTAVAX during the 3 months before getting pregnant.
You may also report these events to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231, or directly to the Vaccine Adverse Event Reporting System (VAERS). The VAERS toll free number is 1-800-822-7967 or report online to www.vaers.hhs.gov.
What are the ingredients of ZOSTAVAX?
Active Ingredient: a weakened form of the varicella-zoster virus.
Inactive Ingredients: sucrose, hydrolyzed porcine gelatin, sodium chloride, monosodium L-glutamate, sodium phosphate dibasic, potassium phosphate monobasic, potassium chloride.
This leaflet summarizes important information about ZOSTAVAX. If you would like more information, talk to your health care provider or visit the website at www.ZOSTAVAX.com or call 1-800-622-4477.
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2006-2018 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
Revised: 08/2018
usppi-v211-i-fro-1808r019
PRINCIPAL DISPLAY PANEL - 0.65 mL Vial Carton
A
NDC 0006-4963-00
1 Single-dose Vial (0.65 mL)
Zoster Vaccine Live
ZOSTAVAX®
STORE FROZEN
Oka/Merck strain. Human
diploid cell (MRC-5) culture
origin. Each 0.65-mL
dose contains a
minimum of 19,400 PFU
(plaque-forming units).
Contains no preservative.
Contains trace quantities
of neomycin.
Rx only

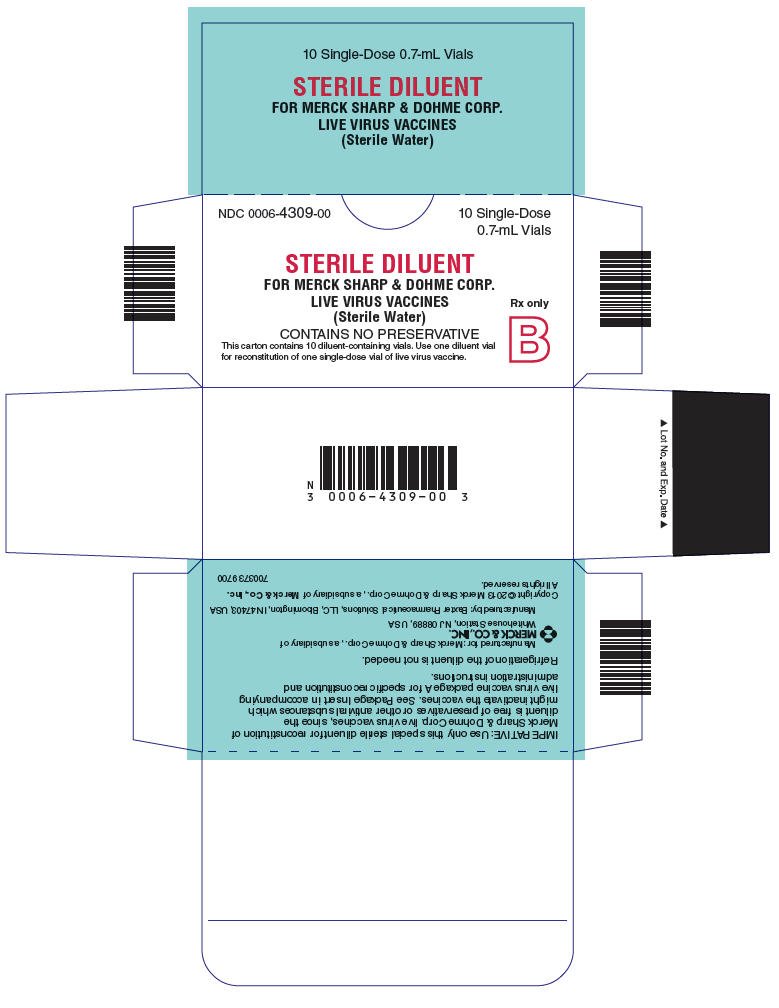
PRINCIPAL DISPLAY PANEL - 0.7 mL Vial Carton
NDC 0006-4309-00
10 Single-Dose
0.7-mL Vials
STERILE DILUENT
FOR MERCK SHARP & DOHME CORP.
LIVE VIRUS VACCINES
(Sterile Water)
Rx only
B
CONTAINS NO PRESERVATIVE
This carton contains 10 diluent-containing vials. Use one diluent vial
for reconstitution of one single-dose vial of live virus vaccine.
| ZOSTAVAX
zoster vaccine live injection, powder, lyophilized, for suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| STERILE DILUENT
sterile water injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |
Frequently asked questions
- Shingrix vs Zostavax - What's the difference between them?
- What are the most common skin conditions? (with photos)
More about Zostavax (zoster vaccine live)
- Check interactions
- Compare alternatives
- Reviews (3)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: viral vaccines
Patient resources
Related treatment guides
Copyright © 2006-2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
