Temozolomide Prescribing Information
Package insert / product label
Dosage form: capsule
Drug class: Alkylating agents
J Code (medical billing code): J8700 (5 mg, oral)
Medically reviewed by Drugs.com. Last updated on Aug 5, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
TEMOZOLOMIDE capsules for oral use
Initial U.S. Approval: 1999
Recent Major Changes
Indications and Usage for Temozolomide
Temozolomide is an alkylating drug indicated for the treatment of adults with:
Temozolomide Dosage and Administration
- Administer orally. (2.4)
-
Newly Diagnosed Glioblastoma:
- 75 mg/m2 once daily for 42 to 49 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1 to 5 of each 28-day cycle for 6 cycles. May increase maintenance dose to 200 mg/m2 for Cycles 2 to 6 based on toxicity. (2.1)
- Provide Pneumocystis pneumonia (PCP) prophylaxis during concomitant phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less. (2.1)
- Adjuvant Treatment of Newly Diagnosed Anaplastic Astrocytoma: Beginning 4 weeks after the end of radiotherapy, administer temozolomide orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage for Cycle 1 is 150 mg/m2 per day and for Cycles 2 to 12 is 200 mg/m2 if patient experienced no or minimal toxicity in Cycle 1. (2.2)
- Refractory Anaplastic Astrocytoma: Initial dose of 150 mg/m2 once daily on Days 1 to 5 of each 28-day cycle. (2.2)
Dosage Forms and Strengths
- Capsules: 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, and 250 mg. (3)
Contraindications
- History of serious hypersensitivity to temozolomide or any other ingredients in temozolomide capsules and dacarbazine. (4)
Warnings and Precautions
- Myelosuppression: Monitor absolute neutrophil count (ANC) and platelet count prior to each cycle and during treatment. Geriatric patients and women have a higher risk of developing myelosuppression. (5.1, 8.5)
- Hepatotoxicity: Fatal and severe hepatotoxicity have been reported. Perform liver tests at baseline, midway through the first cycle, prior to each subsequent cycle, and approximately 2 to 4 weeks after the last dose of temozolomide. (5.2)
- Pneumocystis Pneumonia (PCP): Closely monitor all patients, particularly those receiving steroids, for the development of lymphopenia and PCP. (5.3)
- Secondary Malignancies: Myelodysplastic syndrome and secondary malignancies, including myeloid leukemia, have been observed. (5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. Advise male patients with pregnant partners or female partners of reproductive potential to use condoms. (5.5, 8.1, 8.3)
- Exposure to Opened Capsules: Temozolomide capsules should not be opened, chewed, or dissolved but should be swallowed whole with a glass of water. (5.6)
Adverse Reactions/Side Effects
- The most common adverse reactions (≥20%) are: alopecia, fatigue, nausea, vomiting, headache, constipation, anorexia, and convulsions. (6.1)
- The most common Grade 3 to 4 hematologic laboratory abnormalities (≥10%) in patients with anaplastic astrocytoma are: decreased lymphocytes, decreased platelets, decreased neutrophils, and decreased leukocytes. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
Full Prescribing Information
1. Indications and Usage for Temozolomide
2. Temozolomide Dosage and Administration
2.1 Monitoring to Inform Dosage and Administration
Prior to dosing, withhold temozolomide capsules until patients have an absolute neutrophil count (ANC) of 1.5 x 109/L or greater and a platelet count of 100 x 109/L or greater.
For concomitant radiotherapy, obtain a complete blood count prior to initiation of treatment and weekly during treatment.
For the 28-day treatment cycles, obtain a complete blood count prior to treatment on Day 1 and on Day 22 of each cycle. Perform complete blood counts weekly until recovery if the ANC falls below 1.5 x 109/L and the platelet count falls below 100 x 109/L.
For concomitant use with focal radiotherapy, obtain a complete blood count weekly and as clinically indicated.
2.2 Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma
Administer temozolomide capsules once daily for 42 to 49 consecutive days during the concomitant use phase with focal radiotherapy, and then once daily on Days 1 to 5 of each 28-day cycle for 6 cycles during the maintenance use phase.
Provide Pneumocystis pneumonia (PCP) prophylaxis during the concomitant use phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less [see Warnings and Precautions (5.3)].
Concomitant Use Phase:
The recommended dosage of temozolomide capsules is 75 mg/m2 once daily for 42 to 49 days in combination with focal radiotherapy. Focal radiotherapy includes the tumor bed or resection site with a 2 to 3 cm margin.
Other administration schedules have been used.
Obtain a complete blood count weekly. The recommended dosage modifications due to adverse reactions during concomitant use phase are provided in Table 1.

Single Agent Maintenance Use Phase:
Beginning 4 weeks after concomitant use phase completion, administer temozolomide capsules once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of temozolomide capsules in the maintenance use phase is:
• Cycle 1: 150 mg/m2 per day on days 1 to 5.
• Cycles 2 to 6: May increase to 200 mg/m2 per day on days 1 to 5 before starting Cycle 2 if no dosage interruptions or discontinuations are required (Table 1). If the dose is not escalated at the onset of Cycle 2, do not increase the dose for Cycles 3 to 6.
Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
The recommended dosage modifications due to adverse reactions during the maintenance use phase are provided in Table 2.
If temozolomide capsules are withheld, reduce the dose for the next cycle by 50 mg/m2 per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2 per day.

2.3 Recommended Dosage and Dosage Modifications for Anaplastic Astrocytoma
Adjuvant Treatment of Newly Diagnosed Anaplastic Astrocytoma
Beginning 4 weeks after the end of radiotherapy, administer temozolomide capsules orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage of temozolomide capsules is:
• Cycle 1: 150 mg/m2 per day on days 1 to 5.
• Cycles 2 to 12: 200 mg/m2 per day on days 1 to 5 if patient experienced no or minimal toxicity in Cycle 1. If the dose was not escalated at the onset of Cycle 2, do not increase the dose during Cycles 3 to 6.
The recommended complete blood count testing and dosage modifications due to adverse reactions during adjuvant treatment are provided above and in Table 2 [see Dosage and Administration (2.2)].
Refractory Anaplastic Astrocytoma
The recommended initial dosage of temozolomide capsules is 150 mg/m2 once daily on Days 1 to 5 of each 28-day cycle. Increase the temozolomide dose to 200 mg/m2 per day if the following conditions are met at the nadir and on Day 1 of the next cycle:
• ANC is greater than or equal to 1.5 x 109/L, and
• Platelet count is greater than or equal to 100 x 109/L.
Continue temozolomide capsules until disease progression or unacceptable toxicity.
Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
If the ANC is less than 1 x 109/L or the platelet count is less than 50 x 109/L during any cycle, reduce the temozolomide dose for the next cycle by 50 mg/m2 per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2 per day.
2.4 Preparation and Administration
Temozolomide is a hazardous drug. Follow applicable special handling and disposal procedures.1
Temozolomide capsules
Take temozolomide capsules at the same time each day. Administer temozolomide capsules consistently with respect to food (fasting vs. nonfasting) [see Clinical Pharmacology (12.3)]. To reduce nausea and vomiting, take temozolomide capsules on an empty stomach or at bedtime and consider antiemetic therapy prior to and following temozolomide capsule administration.
Swallow temozolomide capsules whole with water. Advise patients not to open, chew, or dissolve the contents of the capsules [see Warnings and Precautions (5.6)].
If capsules are accidentally opened or damaged, take precautions to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, wash the affected area with water immediately.
3. Dosage Forms and Strengths
- Temozolomide capsules, USP for oral administration
-5 mg capsules have opaque white bodies with opaque light green caps. The capsule body is printed with "604" in black ink and the cap is printed with "LP" in black ink.
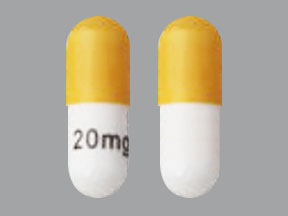
-20 mg capsules have opaque white bodies with opaque yellow caps. The capsule body is printed with "605" in black ink and the cap is printed with "LP" in black ink.
-100 mg capsules have opaque white bodies with opaque pink caps. The capsule body is printed with "606" in black ink and the cap is printed with "LP" in black ink.
-140 mg capsules have opaque white bodies with opaque blue caps. The capsule body is printed with "607" in black ink and the cap is printed with "LP" in black ink.
-180 mg capsules have opaque white bodies with opaque swedish orange caps. The capsule body is printed with "608" in black ink and the cap is printed with "LP" in black ink.
-250 mg capsules have opaque white bodies with opaque white caps. The capsule body is printed with "609" in black ink and the cap is printed with "LP" in black ink.
4. Contraindications
Temozolomide is contraindicated in patients with a history of serious hypersensitivity reactions to:
- temozolomide or any other ingredients in temozolomide capsules; and
- dacarbazine, since both temozolomide and dacarbazine are metabolized to the same active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide.
Reactions to temozolomide have included anaphylaxis [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Myelosuppression
Myelosuppression, including pancytopenia, leukopenia, and anemia, some with fatal outcomes, have occurred with temozolomide [see Adverse Reactions (6.1, 6.2)].
In MK-7365-006, myelosuppression usually occurred during the first few cycles of therapy and was generally not cumulative. The median nadirs occurred at 26 days for platelets (range: 21 to 40 days) and 28 days for neutrophils (range: 1 to 44 days). Approximately 10% of patients required hospitalization, blood transfusion, or discontinuation of therapy due to myelosuppression. Geriatric patients and women have been shown in clinical trials to have a higher risk of developing myelosuppression.
Obtain a complete blood count and monitor ANC and platelet counts before initiation of treatment and as clinically indicated during treatment. When temozolomide is used in combination with radiotherapy, obtain a complete blood count prior to initiation of treatment, weekly during treatment, and as clinically indicated [see Dosage and Administration (2.1, 2.2, 2.3)].
For severe myelosuppression, withhold temozolomide and then resume at same or reduced dose, or permanently discontinue, based on occurrence [see Dosage and Administration (2.1, 2.2, 2.3)].
5.2 Hepatotoxicity
Fatal and severe hepatotoxicity have been reported in patients receiving temozolomide. Perform liver tests at baseline, midway through the first cycle, prior to each subsequent cycle, and approximately two to four weeks after the last dose of temozolomide.
5.3 Pneumocystis Pneumonia
Pneumocystis pneumonia (PCP) has been reported in patients receiving temozolomide. The risk of PCP is increased in patients receiving steroids or with longer treatment regimens of temozolomide.
For patients with newly diagnosed glioblastoma, provide PCP prophylaxis for all patients during the concomitant phase. Continue PCP prophylaxis in patients who experience lymphopenia, until resolution to Grade 1 or less [see Dosage and Administration (2.1)].
Monitor all patients receiving temozolomide for the development of lymphopenia and PCP.
5.4 Secondary Malignancies
The incidence of secondary malignancies is increased in patients treated with temozolomide-containing regimens. Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukemia, have been observed following temozolomide administration.
5.5 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, temozolomide can cause fetal harm when administered to a pregnant woman. Adverse developmental outcomes have been reported in both pregnant patients and pregnant partners of male patients. Oral administration of temozolomide to rats and rabbits during the period of organogenesis resulted in embryolethality and polymalformations at doses less than the maximum human dose based on body surface area.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with temozolomide and for 6 months after the last dose. Because of potential risk of genotoxic effects on sperm, advise male patients with female partners of reproductive potential to use condoms during treatment with temozolomide and for 3 months after the last dose. Advise male patients not to donate semen during treatment with temozolomide and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.6 Exposure to Opened Capsules
Advise patients not to open, chew or dissolve the contents of the temozolomide capsules. Swallow capsules whole with a glass of water. If a capsule becomes damaged, avoid contact of the powder contents with skin or mucous membranes. In case of powder contact, wash affected area with water immediately [see Dosage and Administration (2.4)]. If temozolomide capsules must be opened or the contents must be dissolved, this should be done by a professional trained in safe handling of hazardous drugs using appropriate equipment and safety procedures.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Pneumocystis Pneumonia [see Warnings and Precautions (5.3)]
- Secondary Malignancies [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed Glioblastoma
The safety of temozolomide was evaluated in study MK-7365-051 [see Clinical Studies (14.1)].
Severe or life-threatening adverse reactions occurred in 49% of patients treated with temozolomide; the most common were fatigue (13%), convulsions (6%), headache (5%), and thrombocytopenia (5%).
The most common adverse reactions (≥20%) in patients treated with temozolomide were alopecia, fatigue, nausea, anorexia, headache, constipation, and vomiting.
Table 3 summarizes the adverse reactions in MK-7365-051.

Clinically relevant adverse reactions in <10% of patients are presented below:
Central & Peripheral Nervous System: memory impairment, confusion
Eye: vision blurred
Gastrointestinal System: stomatitis, abdominal pain
General: weakness, dizziness
Immune System: allergic reaction
Injury: radiation injury not otherwise specified
Musculoskeletal System: arthralgia
Platelet, Bleeding, & Clotting: thrombocytopenia
Psychiatric: insomnia
Respiratory System: coughing, dyspnea
Special Senses Other: taste perversion
Skin & Subcutaneous Tissue: dry skin, pruritus, erythema
When laboratory abnormalities and adverse reactions were combined, Grade 3 or Grade 4 neutrophil abnormalities including neutropenic reactions were observed in 8% of patients, and Grade 3 or Grade 4 platelet abnormalities including thrombocytopenic reactions were observed in 14% of patients.
Newly Diagnosed Anaplastic Astrocytoma
The safety of temozolomide for the adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma was derived from published literature [see Clinical Studies (14.2)]. The safety of temozolomide for the adjuvant treatment of patients with newly diagnosed anaplastic astrocytoma was consistent with the known safety profile of temozolomide.
Refractory Anaplastic Astrocytoma
The safety of temozolomide was evaluated in study MK-7365-006 [see Clinical Studies (14.2)].
The most common adverse reactions (≥20%) were nausea, vomiting, headache, fatigue, constipation, and convulsions.
Tables 4 and 5 summarize the adverse reactions and hematological laboratory abnormalities in MK-7365-006.

Clinically relevant adverse reactions in <10% of patients are presented below:
Central and Peripheral Nervous System: paresthesia, somnolence, paresis, urinary incontinence, ataxia, dysphasia, convulsions local, gait abnormal, confusion
Endocrine: adrenal hypercorticism
Gastrointestinal System: abdominal pain, anorexia
General: back pain
Metabolic: weight increase
Musculoskeletal System: myalgia
Psychiatric: anxiety, depression
Reproductive Disorders: breast pain female
Respiratory System: upper respiratory tract infection, pharyngitis, sinusitis, coughing
Skin & Appendages: rash, pruritus
Urinary System: urinary tract infection, micturition increased frequency
Vision: diplopia, vision abnormal*
* This term includes blurred vision; visual deficit; vision changes; and vision troubles.

Hematological Toxicities for Advanced Gliomas
In clinical trial experience with 110 to 111 females and 169 to 174 males (depending on measurements), females experienced higher rates of Grade 4 neutropenia (ANC <0.5 x 109/L) and thrombocytopenia (<20 x 109/L) than males in the first cycle of therapy (12% vs. 5% and 9% vs. 3%, respectively).
In the entire safety database for which hematologic data exist (N=932), 7% (4/61) and 10% (6/63) of patients >70 years experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. For patients ≤70 years, 7% (62/871) and 6% (48/879) experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. Pancytopenia, leukopenia, and anemia also occurred.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of temozolomide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.
Dermatologic: Toxic epidermal necrolysis and Stevens-Johnson syndrome.
Immune System: Hypersensitivity reactions, including anaphylaxis. Erythema multiforme, which resolved after discontinuation of temozolomide and, in some cases, recurred upon rechallenge.
Hematopoietic: Prolonged pancytopenia, which may result in aplastic anemia and fatal outcomes.
Hepatobiliary: Fatal and severe hepatotoxicity, elevation of liver enzymes, hyperbilirubinemia, cholestasis, and hepatitis.
Infections: Serious opportunistic infections, including some cases with fatal outcomes, with bacterial, viral (primary and reactivated), fungal, and protozoan organisms.
Pulmonary: Interstitial pneumonitis, pneumonitis, alveolitis, and pulmonary fibrosis.
Endocrine: Diabetes insipidus.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], temozolomide can cause fetal harm when administered to a pregnant woman. Available postmarketing reports describe cases of spontaneous abortions and congenital malformations, including polymalformations with central nervous system, facial, cardiac, skeletal, and genitourinary system anomalies with exposure to temozolomide during pregnancy. These cases report similar adverse developmental outcomes to those observed in animal studies. Administration of temozolomide to rats and rabbits during the period of organogenesis caused numerous external, internal, and skeletal malformations at doses less than the maximum human dose based on body surface area (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Five consecutive days of oral administration of temozolomide at doses of 75 and 150 mg/m2 (0.38 and 0.75 times the human dose of 200 mg/m2) in rats and rabbits, respectively, during the period of organogenesis (Gestation Days 8 to 12) caused numerous malformations of the external and internal organs and skeleton in both species. In rabbits, temozolomide at the 150 mg/m2 dose (0.75 times the human dose of 200 mg/m2) caused embryolethality as indicated by increased resorptions.
8.2 Lactation
There are no data on the presence of temozolomide or its metabolites in human milk, the effects on a breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions, including myelosuppression from temozolomide in the breastfed children, advise women not to breastfeed during treatment with temozolomide and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Temozolomide can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating temozolomide [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with temozolomide and for 6 months after the last dose.
Males
Because of the potential for embryofetal toxicity and genotoxic effects on sperm cells, advise male patients with pregnant partners or female partners of reproductive potential to use condoms during treatment with temozolomide and for 3 months after the last dose [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)].
Advise male patients not to donate semen during treatment with temozolomide and for 3 months after the last dose.
Infertility
Temozolomide may impair male fertility [see Nonclinical Toxicology (13.1)]. Limited data from male patients show changes in sperm parameters during treatment with temozolomide; however, no information is available on the duration or reversibility of these changes.
8.4 Pediatric Use
Safety and effectiveness of temozolomide have not been established in pediatric patients. Safety and effectiveness of temozolomide capsules were assessed, but not established, in 2 open-label studies in pediatric patients aged 3 to 18 years. In one study, 29 patients with recurrent brain stem glioma and 34 patients with recurrent high-grade astrocytoma were enrolled. In a second study conducted by the Children’s Oncology Group (COG), 122 patients were enrolled, including patients with medulloblastoma/PNET (29), high grade astrocytoma (23), low grade astrocytoma (22), brain stem glioma (16), ependymoma (14), other CNS tumors (9), and non-CNS tumors (9). The adverse reaction profile in pediatric patients was similar to adults.
8.5 Geriatric Use
In MK-7365-051, 15% of patients with newly diagnosed glioblastoma were 65 years and older. This study did not include sufficient numbers of patients aged 65 years and older to determine differences in effectiveness from younger patients. No overall differences in safety were observed between patients ≥65 years and younger patients.
The CATNON trial did not include sufficient numbers of patients aged 65 years and older to determine differences in safety or effectiveness when compared to younger patients.
In MK-7365-006, 4% of patients with refractory anaplastic astrocytoma were 70 years and older. This study did not include sufficient numbers of patients aged 70 years and older to determine differences in effectiveness from younger patients. Patients 70 years and older had a higher incidence of Grade 4 neutropenia (25%) and Grade 4 thrombocytopenia (20%) in the first cycle of therapy than patients less than 70 years of age [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
In the entire safety database for which hematologic data exist (N=932), 7% (4/61) and 10% (6/63) of patients >70 years experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. For patients ≤70 years, 7% (62/871) and 6% (48/879) experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. Pancytopenia, leukopenia, and anemia also occurred.
8.6 Renal Impairment
No dosage adjustment is recommended for patients with creatinine clearance (CLcr) of 36 to 130 mL/min/m2[see Clinical Pharmacology (12.3)]. The recommended dose of temozolomide has not been established for patients with severe renal impairment (CLcr <36 mL/min/m2) or for patients with end-stage renal disease on dialysis.
8.7 Hepatic Impairment
No dosage adjustment is recommended for patients with mild to moderate hepatic impairment (Child Pugh class A and B) [see Clinical Pharmacology (12.3)]. The recommended dose of temozolomide has not been established for patients with severe hepatic impairment (Child-Pugh class C).
10. Overdosage
Dose-limiting toxicity was myelosuppression and was reported with any dose but is expected to be more severe at higher doses. An overdose of 2000 mg per day for 5 days was taken by one patient and the adverse reactions reported were pancytopenia, pyrexia, multi-organ failure, and death. There are reports of patients who have taken more than 5 days of treatment (up to 64 days), with adverse reactions reported including myelosuppression, which in some cases was severe and prolonged, and infections and resulted in death. In the event of an overdose, monitor complete blood count and provide supportive measures as necessary.
11. Temozolomide Description
Temozolomide is an alkylating drug. The chemical name of temozolomide is 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide. The structural formula of temozolomide is:

The material is a white to light tan/light pink powder with a molecular formula of C6H6N6O2 and a molecular weight of 194.15. The molecule is stable at acidic pH (<5) and labile at pH >7; hence temozolomide can be administered orally and intravenously. The prodrug, temozolomide, is rapidly hydrolyzed to the active 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC) at neutral and alkaline pH values, with hydrolysis taking place even faster at alkaline pH.
Temozolomide capsules, USP:
Each capsule for oral use contains either 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, or 250 mg of temozolomide.
The inactive ingredients for Temozolomide Capsules, USP are as follows:
Temozolomide Capsules 5 mg: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, and stearic acid.
Temozolomide Capsules 20 mg: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, and stearic acid.
Temozolomide Capsules 100 mg: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, and stearic acid.
Temozolomide Capsules 140 mg: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, and stearic acid.
Temozolomide Capsules 180 mg: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, and stearic acid.
Temozolomide Capsules 250 mg: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, and stearic acid.
The body of the capsules is made of gelatin and is opaque white. The cap is also made of gelatin, and the colors vary based on the dosage strength. The capsule body and cap are imprinted with pharmaceutical branding ink, which contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide, and ferric oxide.
Temozolomide Capsules 5 mg: The green cap contains gelatin, titanium dioxide, yellow iron oxide, and FD&C Blue 2.
Temozolomide Capsules 20 mg: The yellow cap contains gelatin, titanium dioxide, and yellow iron oxide.
Temozolomide Capsules 100 mg: The pink cap contains gelatin, titanium dioxide, and yellow iron oxide, and red iron oxide.
Temozolomide Capsules 140 mg: The blue cap contains gelatin, titanium dioxide, yellow iron oxide and FD&C Blue #2.
Temozolomide Capsules 180 mg: The orange cap contains gelatin, titanium dioxide and red iron oxide.
Temozolomide Capsules 250 mg: The white cap contains gelatin, titanium dioxide.
FDA approved dissolution test specifications differ from USP.
12. Temozolomide - Clinical Pharmacology
12.1 Mechanism of Action
Temozolomide is not directly active but undergoes rapid nonenzymatic conversion at physiologic pH to the reactive compound 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC). The cytotoxicity of MTIC is thought to be primarily due to DNA alkylation, mainly at the O6 and N7 positions of guanine, which causes DNA double strand breaks and results in programmed cell death.
12.2 Pharmacodynamics
Temozolomide exposure-response relationships and the time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
Following a single oral dose of 150 mg/m2, the mean Cmax is 7.5 mcg/mL for temozolomide and 282 ng/mL for MTIC. The mean AUC is 23.4 mcg·hr/mL for temozolomide and 864 ng·hr/mL for MTIC.
Following a single 90-minute intravenous infusion of 150 mg/m2, the mean Cmax is 7.3 mcg/mL for temozolomide and 276 ng/mL for MTIC. The mean AUC is 24.6 mcg·hr/mL for temozolomide and 891 ng·hr/mL for MTIC.
Temozolomide exhibits linear kinetics over the therapeutic dosing range of 75 mg/m2/day to 250 mg/m2/day.
Absorption
The median Tmax is 1 hour.
Effect of Food
The mean temozolomide Cmax and AUC decreased by 32% and 9%, respectively, and median Tmax increased by 2-fold (from 1 to 2.25 hours) when temozolomide capsules were administered after a modified high-fat breakfast (587 calories comprised of 1 fried egg, 2 strips of bacon, 2 slices of toast, 2 pats of butter, and 8 oz whole milk).
Distribution
Temozolomide has a mean (CV%) apparent volume of distribution of 0.4 L/kg (13%). The mean percent bound of drug-related total radioactivity is 15%.
Elimination
Clearance of temozolomide is approximately 5.5 L/hr/m2 and the mean elimination half-life is 1.8 hours.
Metabolism
Temozolomide is spontaneously hydrolyzed at physiologic pH to the active species, MTIC and to temozolomide acid metabolite. MTIC is further hydrolyzed to 5-amino-imidazole-4-carboxamide (AIC), which is known to be an intermediate in purine and nucleic acid biosynthesis, and to methylhydrazine, which is believed to be the active alkylating species. Cytochrome P450 enzymes play a minor role in the metabolism of temozolomide and MTIC. Relative to the AUC of temozolomide, the exposure to MTIC and AIC is 2.4% and 23%, respectively.
Excretion
Approximately 38% of the administered temozolomide total radioactive dose is recovered over 7 days: 38% in urine and 0.8% in feces. The majority of the recovered radioactivity in urine is unchanged temozolomide (6%), AIC (12%), temozolomide acid metabolite (2.3%), and unidentified polar metabolite(s) (17%).
Specific Populations
No clinically significant differences in the pharmacokinetics of temozolomide were observed based on age (range: 19 to 78 years), gender, smoking status (smoker vs. non-smoker), creatinine clearance (CLcr) of 36 to 130 mL/min/m2, or mild to moderate hepatic impairment (Child Pugh class A and B). The pharmacokinetics of temozolomide has not been studied in patients with CLcr <36 mL/min/m2, end-stage renal disease on dialysis, or severe hepatic impairment (Child-Pugh class C).
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
No clinically significant differences in the pharmacokinetics of temozolomide or MTIC were observed when co-administered with ranitidine.
No clinically significant differences in the clearance of temozolomide or MTIC were predicted when co-administered with the following drugs: valproic acid, dexamethasone, prochlorperazine, phenytoin, carbamazepine, ondansetron, histamine-2-receptor antagonists, or phenobarbital.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Temozolomide is carcinogenic in rats at doses less than the maximum recommended human dose. Temozolomide induced mammary carcinomas in both males and females at doses 0.13 to 0.63 times the maximum human dose (25 to 125 mg/m2) when administered orally on 5 consecutive days every 28 days for 6 cycles. Temozolomide also induced fibrosarcomas of the heart, eye, seminal vesicles, salivary glands, abdominal cavity, uterus, and prostate, carcinomas of the seminal vesicles, schwannomas of the heart, optic nerve, and harderian gland, and adenomas of the skin, lung, pituitary, and thyroid at doses 0.5 times the maximum daily dose. Mammary tumors were also induced following 3 cycles of temozolomide at the maximum recommended daily dose.
Temozolomide is a mutagen and a clastogen. In a reverse bacterial mutagenesis assay (Ames assay), temozolomide increased revertant frequency in the absence and presence of metabolic activation. Temozolomide was clastogenic in human lymphocytes in the presence and absence of metabolic activation.
Temozolomide impairs male fertility. Temozolomide caused syncytial cells/immature sperm formation at doses of 50 and 125 mg/m2 (0.25 and 0.63 times the human dose of 200 mg/m2) in rats and dogs, respectively, and testicular atrophy in dogs at 125 mg/m2.
13.2 Animal Toxicology and/or Pharmacology
Toxicology studies in rats and dogs identified a low incidence of hemorrhage, degeneration, and necrosis of the retina at temozolomide doses equal to or greater than 125 mg/m2 (0.63 times the human dose of 200 mg/m2). These changes were most commonly seen at doses where mortality was observed.
14. Clinical Studies
14.1 Newly Diagnosed Glioblastoma
The efficacy of temozolomide was evaluated in MK-7365-051 (NCT00006353), a randomized (1:1), multicenter, open-label trial. Eligible patients were required to have newly diagnosed glioblastoma. Patients were randomized to receive either radiation therapy alone or concomitant temozolomide 75 mg/m2 once daily starting the first day of radiation therapy and continuing until the last day of radiation therapy for 42 days (with a maximum of 49 days), followed by temozolomide 150 mg/m2 or 200 mg/m2 once daily on Days 1 to 5 of each 28-day cycle, starting 4 weeks after the end of radiation therapy and continuing for 6 cycles. In both arms, focal radiation therapy was delivered as 60 Gy/30 fractions and included radiation to the tumor bed or resection site with a 2- to 3-cm margin. PCP prophylaxis was required during the concomitant phase regardless of lymphocyte count and continued until recovery of lymphocyte count to Grade 1 or less. The major efficacy outcome measure was overall survival.
A total of 573 patients were randomized, 287 to temozolomide and radiation therapy and 286 to radiation therapy alone. At the time of disease progression, temozolomide was administered as salvage therapy in 161 patients of the 282 (57%) in the radiation therapy alone arm and 62 patients of the 277 (22%) in the temozolomide and radiation therapy arm.
The addition of concomitant and maintenance temozolomide to radiation therapy for the treatment of patients with newly diagnosed glioblastoma showed a statistically significant improvement in overall survival compared to radiotherapy alone (Figure 1). The hazard ratio (HR) for overall survival was 0.63 (95% CI: 0.52, 0.75) with a log-rank P<0.0001 in favor of the temozolomide arm. The median overall survival was 14.6 months in the temozolomide arm and 12.1 months for radiation therapy alone arm.
 |
14.2 Anaplastic Astrocytoma
Newly Diagnosed Anaplastic Astrocytoma
The efficacy of temozolomide for the adjuvant treatment of newly diagnosed anaplastic astrocytoma was derived from studies of temozolomide in the published literature. Temozolomide was evaluated in CATNON (NCT00626990), a randomized, open-label, multicenter trial, where the major efficacy outcome measure was overall survival.
Refractory Anaplastic Astrocytoma
The efficacy of temozolomide was evaluated in Study MK-7365-006, a single-arm, multicenter trial. Eligible patients had anaplastic astrocytoma at first relapse and a baseline Karnofsky performance status (KPS) of 70 or greater. Patients had previously received radiation therapy and may also have previously received a nitrosourea with or without other chemotherapy. Fifty-four patients had disease progression on prior therapy with both a nitrosourea and procarbazine and their malignancy was considered refractory to chemotherapy (refractory anaplastic astrocytoma population). Temozolomide capsules were given on Days 1 to 5 of each 28-day cycle at a starting dose of 150 mg/m2/day. If ANC was ≥1.5 x 109/L and platelet count was ≥100 x 109/L at the nadir and on Day 1 of the next cycle, the temozolomide dose was increased to 200 mg/m2/day. The major efficacy outcome measure was progression-free survival at 6 months and the additional efficacy outcome measures were overall survival and overall response rate.
In the refractory anaplastic astrocytoma population (n=54), the median age was 42 years (range: 19 to 76); 65% were male; and 72% had a KPS of >80. Sixty-three percent of patients had surgery other than a biopsy at the time of initial diagnosis. Of those patients undergoing resection, 73% underwent a subtotal resection and 27% underwent a gross total resection. Eighteen percent of patients had surgery at the time of first relapse. The median time from initial diagnosis to first relapse was 13.8 months (range: 4.2 months to 6.3 years).
In the refractory anaplastic astrocytoma population, the overall response rate (CR+PR) was 22% (12 of 54 patients) and the complete response rate was 9% (5 of 54 patients). The median duration of all responses was 50 weeks (range: 16 to 114 weeks) and the median duration of complete responses was 64 weeks (range: 52 to 114 weeks). In this population, progression-free survival at 6 months was 45% (95% CI: 31%, 58%) and progression-free survival at 12 months was 29% (95% CI: 16%, 42%). Median progression-free survival was 4.4 months. Overall survival at 6 months was 74% (95% CI: 62%, 86%) and 12-month overall survival was 65% (95% CI: 52%, 78%). Median overall survival was 15.9 months.
16. How is Temozolomide supplied
Temozolomide is a hazardous drug. Follow applicable special handling and disposal procedures.1
Temozolomide Capsules, USP are supplied in HDPE plastic bottles with child-resistant polypropylene caps containing the following capsule strengths:
Temozolomide Capsules, USP 5 mg: have opaque white bodies with opaque light green caps. The capsule body is printed with "604" in black ink and the cap is printed with "LP" in black ink. They are supplied as follows:
5 count: NDC 16571-816-51
14 count: NDC 16571-816-41
20 count: NDC 16571-816-02
Temozolomide Capsules, USP 20 mg: have opaque white bodies with opaque rich yellow caps. The capsule body is printed with “605” in black ink and the cap is printed with “LP” in black ink. They are supplied as follows:
5 count: NDC 16571-817-51
14 count: NDC 16571-817-41
20 count: NDC 16571-817-02
Temozolomide Capsules, USP 100 mg: have opaque white bodies with opaque pink caps. The capsule body is printed with "606" in black ink and the cap is printed with "LP" in black ink. They are supplied as follows:
5 count: NDC 16571-818-51
14 count: NDC 16571-818-41
20 count: NDC 16571-818-02
Temozolomide Capsules, USP 140 mg: have opaque white bodies with opaque powder blue caps. The capsule body is printed with "607" in black ink and the cap is printed with "LP" in black ink. They are supplied as follows:
5 count: NDC 16571-819-51
14 count: NDC 16571-819-41
20 count: NDC 16571-819-02
Temozolomide Capsules, USP 180 mg: have opaque white bodies with opaque swedish orange caps. The capsule body is printed with "608" in black ink and the cap is printed with "LP" in black ink. They are supplied as follows:
5 count: NDC 16571-820-51
14 count: NDC 16571-820-41
20 count: NDC 16571-820-02
Temozolomide Capsules, USP 250 mg: have opaque white bodies with opaque white caps. The capsule body is printed with “609” in black ink and the cap is printed with “LP” in black ink. They are supplied as follows:
5 count: NDC 16571-821-51
20 count: NDC 16571-821-02
Store Temozolomide Capsules at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myelosuppression
Inform patients that temozolomide capsules can cause low blood cell counts and the need for frequent monitoring of blood cell counts. Advise patients to contact their healthcare provider immediately for bleeding, fever, or other signs of infection [see Warnings and Precautions (5.1)].
Hepatotoxicity
Advise patients of the increased risk of hepatotoxicity and to contact their healthcare provider immediately for signs or symptoms of hepatotoxicity. Inform patients that they will have periodic liver enzyme tests during treatment and following the last dose of temozolomide capsules [see Warnings and Precautions (5.2)].
Pneumocystis Pneumonia
Advise patients of the increased risk of Pneumocystis pneumonia and to contact their healthcare provider immediately for new or worsening pulmonary symptoms. Inform patients that prophylaxis for Pneumocystis pneumonia may be needed [see Dosage and Administration (2.1), Warnings and Precautions (5.3)].
Secondary Malignancies
Advise patients of the increased risk of myelodysplastic syndrome and secondary malignancies [see Warnings and Precautions (5.4)].
Exposure to Opened Capsules
Advise patient to not open, chew, or dissolve the capsules. If capsules are accidentally opened or damaged, advise patients to take rigorous precautions with capsule contents to avoid inhalation or contact with the skin or mucous membranes [see Warnings and Precautions (5.6)]. In case of powder contact, wash the affected area with water immediately [see Dosage and Administration (2.4)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with temozolomide and for 6 months after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with pregnant partners or female partners of reproductive potential to use condoms during treatment with temozolomide and for 3 months after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Advise male patients not to donate semen during treatment with temozolomide and for 3 months after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with temozolomide and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males of reproductive potential that temozolomide may impair fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Code No.: TS/DRUGS/24/2015
Revised: 07/2024
PIR82120-03
Patient Information
Temozolomide (te-moe-ZOE-loe-mide) Capsules, USP
What are temozolomide capsules?
Temozolomide capsules are a prescription medicine used to treat adults with certain brain cancer tumors.
It is not known if temozolomide capsules are safe and effective in children.
Do not take temozolomide capsules if you:
- have had an allergic reaction to temozolomide or any of the other ingredients in temozolomide capsules. See the end of this leaflet for a list of ingredients in temozolomide capsules. Symptoms of an allergic reaction with temozolomide capsules may include: a red itchy rash, or a severe allergic reaction, such as trouble breathing, swelling of the face, throat, or tongue, or severe skin reaction. If you are not sure, ask your healthcare provider.
- have had an allergic reaction to dacarbazine (DTIC), another cancer medicine.
Before taking or receiving temozolomide capsules, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems
- have liver problems
- are pregnant or plan to become pregnant. Temozolomide capsule can harm your unborn baby and cause birth defects.
Females who can become pregnant:
o You should not become pregnant during treatment with temozolomide capsules.
o You should use an effective form of birth control (contraception) during treatment and for 6 months after your last dose of temozolomide capsules.
o Your healthcare provider should do a pregnancy test to make sure that you are not pregnant before you start taking temozolomide capsules.
o Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with temozolomide capsules.
Males with a female partner who is pregnant or who can become pregnant:
o Use a condom for birth control (contraception) during treatment and for 3 months after taking your last dose of temozolomide capsules.
o Do not donate semen during treatment and for 3 months after your last dose of temozolomide capsules.
- are breastfeeding or plan to breastfeed. It is not known if temozolomide passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose of temozolomide capsules.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take temozolomide capsules?
Temozolomide may be taken 2 different ways:
- you may take temozolomide by mouth as a capsule, or
- you may receive temozolomide as an intravenous (IV) injection into your vein.
Your healthcare provider will decide the best way for you to take temozolomide.
- If your healthcare provider prescribes temozolomide capsules for you, take the capsules exactly as prescribed.
There are 2 common dosing schedules for taking or receiving temozolomide capsules depending on the type of brain cancer tumor that you have.
- People with certain brain cancer tumors take or receive temozolomide capsules:
- 1 time each day for 42 to 49 days in a row, along with receiving radiation treatment. This is 1 cycle of treatment. After this, your healthcare provider may prescribe 6 more cycles of temozolomide as “maintenance” treatment. For each of these cycles, you take or receive temozolomide 1 time each day for 5 days in a row and then you stop taking it for the next 23 days. This is a 28-day maintenance treatment cycle.
- People with certain other brain cancer tumors take or receive temozolomide capsules:
- 1 time each day for 5 days in a row only, and then stop taking it for the next 23 days. This is 1 cycle of treatment (28 days).
- Your healthcare provider will watch your progress on temozolomide and decide how long you should take it.
- If your healthcare provider prescribes a treatment regimen that is different from the information in this leaflet, make sure you follow the instructions given to you by your healthcare provider.
- Your healthcare provider may change your dose of temozolomide, or tell you to stop temozolomide capsules for a short period of time or permanently if you have certain side effects.
- Your healthcare provider will decide how many treatment cycles of temozolomide that you will receive, depending on how you respond to and tolerate treatment.
Temozolomide capsules:
- Take temozolomide capsules exactly as your healthcare provider tells you to.
- Temozolomide capsules contain a white capsule body with a color cap and the colors vary based on the dosage strength. Your healthcare provider may prescribe more than 1 strength of temozolomide capsules for you, so it is important that you understand how to take your medicine the right way. Be sure that you understand exactly how many capsules you need to take on each day of your treatment, and what strengths to take. This may be different whenever you start a new cycle.
- Do not take more temozolomide capsules than prescribed.
- Talk to your healthcare provider or pharmacist before taking your dose if you are not sure how much temozolomide capsules to take. This will help to prevent taking too many temozolomide capsules and decrease your chances of getting serious side effects.
- Take each day’s dose of temozolomide capsules at one time, with a full glass of water.
- Take temozolomide capsules at the same time each day.
- Take temozolomide capsules the same way each time, either with food or without food.
- Swallow temozolomide capsules whole with water. Do not open, chew, or dissolve the contents of the capsules.
- If temozolomide capsules are accidentally opened or damaged, be careful not to breathe in (inhale) the powder from the capsules or get the powder on your skin or mucous membranes (for example, in your nose or mouth). If contact with any of these areas happens, wash the area with water right away.
- To help reduce nausea and vomiting, try to take temozolomide capsules on an empty stomach or at bedtime. Your healthcare provider may prescribe medicine to help prevent or treat nausea, or other medicines to reduce side effects with temozolomide capsules.
- See your healthcare provider regularly to check your progress. Your healthcare provider will check you for side effects.
- If you take more temozolomide capsules than prescribed, call your healthcare provider or get emergency medical help right away.
What are the possible side effects of temozolomide capsules?
Temozolomide capsules can cause serious side effects, including:
-
Decreased blood cell counts. Temozolomide capsules can affect your bone marrow and cause you to have decreased blood cell counts. Decreased white blood cell count, red blood cell count and platelet count are common with temozolomide capsules but it can also be severe and lead to death. Some people need to be hospitalized or need to receive transfusions to treat their decreased blood cell counts.
- Your healthcare provider will do blood tests regularly to check your blood cell counts before you start and during treatment with temozolomide capsules.
- Your healthcare provider may need to change the dose of temozolomide capsules or when you get it depending on your blood cell counts.
- People who are age 70 or older and women have a higher risk for developing decreased blood cell counts during treatment with temozolomide capsules.
- Liver problems. Liver problems can happen with temozolomide capsules and can sometimes be severe and lead to death. Your healthcare provider will do blood tests to check your liver function before you start taking temozolomide capsules, during treatment, and about 2 to 4 weeks after your last dose of temozolomide capsules.
-
Pneumocystis pneumonia (PCP). PCP is an infection that people can get when their immune system is weak. Temozolomide capsules decreases white blood cells, which makes your immune system weaker and can increase your risk of getting PCP.
- People who are taking steroid medicines or who stay on temozolomide capsules for a longer period of time may have an increased risk of getting PCP infection.
- Anyone who takes temozolomide capsules will be watched carefully by their healthcare provider for low blood cell counts and this infection.
- Tell your healthcare provider if you have any of the following signs and symptoms of PCP infection: shortness of breath, or fever, chills, dry cough.
- Secondary Cancers. Blood problems such as myelodysplastic syndrome (MDS) and new cancers (secondary cancers), including a certain kind of leukemia, can happen in people who take temozolomide capsules. Your healthcare provider will monitor you for this.
Common side effects of temozolomide capsules include:
- hair loss
- feeling tired
- nausea and vomiting
- headache
- constipation
- loss of appetite
- convulsions
Temozolomide capsules can affect fertility in males and may affect your ability to father a child. Talk with your healthcare provider if fertility is a concern for you.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of temozolomide capsules. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store temozolomide capsules?
• Store temozolomide capsules at room temperature between 68°F to 77°F (20°C to 25°C).
• Keep temozolomide capsules and all medicines out of the reach of children.
General information about the safe and effective use of temozolomide capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use temozolomide capsules for a condition for which it was not prescribed. Do not give temozolomide capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about temozolomide capsules that is written for health professionals.
For more information, contact Rising Pharma Holdings, Inc. at 1-844-874-7464.
What are the ingredients in temozolomide capsules?
Temozolomide capsules:
Active ingredient: temozolomide
Inactive ingredients: lactose anhydrous, colloidal silicon dioxide, sodium starch glycolate, tartaric acid, stearic acid.
The body of the capsules is made of gelatin and is opaque white. The cap is also made of gelatin, and the colors vary based on the dosage strength. The capsule body and cap are imprinted with pharmaceutical branding ink, which contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia, potassium hydroxide, and ferric oxide.
Temozolomide 5 mg: The green cap contains gelatin, titanium dioxide, yellow iron oxide, and FD&C Blue 2.
Temozolomide 20 mg: The yellow cap contains gelatin, titanium dioxide, and yellow iron oxide.
Temozolomide 100 mg: The pink cap contains gelatin, titanium dioxide, and yellow iron oxide, and red iron oxide.
Temozolomide 140 mg: The blue cap contains gelatin, titanium dioxide, yellow iron oxide and FD&C Blue #2.
Temozolomide 180 mg: The orange cap contains gelatin, titanium dioxide and red iron oxide.
Temozolomide 250 mg: The white cap contains gelatin, titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Code No.: TS/DRUGS/24/2015
Revised: 03/2024
PILR82120-01
Temozolomide (te-moe-ZOE-loe-mide) Capsules, USP
PHARMACIST:
Dispense enclosed Patient Package Insert to each patient.
|
IMPORTANT DISPENSING INFORMATION For every patient, dispense temozolomide capsules in its original package, making sure each container lists the strength per capsule and that patients take the appropriate number of capsules from each package. Please see the dispensing instructions below for more information. |
What is temozolomide capsule? [See Full Prescribing Information, Indications and Usage (1).]
Temozolomide is an oral alkylating agent for the treatment of newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytoma.
How is temozolomide capsule dosed? [See Full Prescribing Information, Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma (2.1), Recommended Dosage and Dosage Modifications for Refractory Anaplastic Astrocytoma (2.2).]
The physician calculates the daily dose of temozolomide capsules for a given patient based on the patient's body surface area (BSA). Round off the resulting dose to the nearest 5 mg. An example of the dosing may be as follows: the initial daily dose of temozolomide capsules in milligrams is the BSA multiplied by mg/m2/day (e.g., a patient with a BSA of 1.84 is 1.84 x 75 mg = 138, or 140 mg/day). Adjust the dose for subsequent cycles according to nadir neutrophil and platelet counts in the previous cycle and at the time of initiating the next cycle.
How might the dose of temozolomide capsules be modified for Refractory Anaplastic Astrocytoma? [See Full Prescribing Information, Recommended Dosage and Dosage Modifications for Refractory Anaplastic Astrocytoma (2.2).]
The initial dose is 150 mg/m2 orally once daily for 5 consecutive days per 28-day treatment cycle. Increase the temozolomide capsules dose to 200 mg/m2/day for 5 consecutive days per 28-day treatment cycle if both the nadir and day of dosing (Day 29, Day 1 of next cycle) absolute neutrophil counts (ANC) are greater than or equal to 1.5 x 109/L (1,500/µL) and both the nadir and Day 29, Day 1 of next cycle platelet counts are greater than or equal to 100 x 109/L (100,000/µL). During treatment, obtain a complete blood count on Day 22 (21 days after the first dose), and weekly until the ANC is above 1.5 x 109/L (1,500/µL) and the platelet count exceeds 100 x 109/L (100,000/µL). Do not start the next cycle of temozolomide until the ANC and platelet count exceed these levels. If the ANC falls to less than 1.0 x 109/L (1,000/µL) or the platelet count is less than 50 x 109/L (50,000/µL) during any cycle, reduce the dose for the next cycle by 50 mg/m2. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2 per day.
Patients should continue taking temozolomide capsules until their physician determines that their disease has progressed or until unacceptable side effects or toxicities occur. In the clinical trial, treatment could be continued for a maximum of 2 years, but the optimum duration of therapy is not known. Physicians may alter the treatment regimen for a given patient.
Dosing for Patients with Newly Diagnosed Glioblastoma Multiforme [See Full Prescribing Information, Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma (2.1).]
Concomitant Phase Treatment Schedule
Administer temozolomide capsules orally at 75 mg/m2 daily for 42 days concomitant with focal radiotherapy (60 Gy administered in 30 fractions), followed by maintenance temozolomide capsules for 6 cycles. No dose reductions are recommended; however, dose interruptions may occur based on patient tolerance. Continue the temozolomide capsules dose throughout the 42-day concomitant period up to 49 days if all of the following conditions are met: absolute neutrophil count greater than or equal to 1.5 x 109/L, platelet count greater than or equal to 100 x109/L, and nonhematological adverse reactions less than or equal to Grade 1 (except for alopecia, nausea and vomiting). During treatment, obtain a complete blood count weekly. Interrupt or discontinue temozolomide dosing during the concomitant phase according to the hematological and nonhematological toxicity criteria [see Table 1 in the Full Prescribing Information, Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma (2.1)]. Pneumocystis pneumonia (PCP) prophylaxis is required during the concomitant administration of temozolomide capsules and radiotherapy, and should be continued in patients who develop lymphocytopenia until resolution to Grade 1 or less.
Maintenance Phase Treatment Schedule
Four weeks after completing the temozolomide capsules and radiotherapy phase, administer temozolomide for an additional 6 cycles of maintenance treatment. Dosage in Cycle 1 (maintenance) is 150 mg/m2 once daily for 5 days followed by 23 days without treatment. At the start of Cycle 2, escalate the dose to 200 mg/m2, if the nonhematologic adverse reactions for Cycle 1 are Grade less than or equal to 2 (except for alopecia, nausea and vomiting), absolute neutrophil count (ANC) is greater than or equal to 1.5 x 109/L, and the platelet count is greater than or equal to 100 x 109/L. If the dose was not escalated at Cycle 2, do not escalate the dose in subsequent cycles. Maintain the dose at 200 mg/m2 per day for the first 5 days of each subsequent cycle except if toxicity occurs.
During treatment, obtain a complete blood count on Day 22 (21 days after the first dose) and weekly until the ANC is above 1.5 x 109/L (1,500/μL) and the platelet count exceeds 100 x 109/L (100,000/μL). Do not start the next cycle of temozolomide capsules until the ANC and platelet count exceed these levels. Base dose reductions during the next cycle on the lowest blood counts and worst nonhematologic adverse reactions during the previous cycle. Apply dose reductions or discontinuations during the maintenance phase [see Table 2 in the Full Prescribing Information, Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma (2.1)].
How is temozolomide capsules taken? [See Full Prescribing Information, Preparation and Administration, temozolomide capsules (2.3).]
Advise patients to take each day's dose with a full glass of water, preferably on an empty stomach or at bedtime. Taking the medication on an empty stomach or at bedtime may help ease nausea. If patients are also taking anti-nausea or other medications to relieve the side effects associated with temozolomide capsules, advise them to take these medications prior to and/or following administration of temozolomide capsules. Advise patients that temozolomide capsules should be swallowed whole and NEVER CHEWED. Advise patients that they SHOULD NOT open or split the capsules. If capsules are accidentally opened or damaged, advise patients to take rigorous precautions with the capsule contents to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, advise the patients to wash their hands. Advise patients to keep this medication away from children.
What should the patient avoid during treatment with temozolomide capsules? [See Full Prescribing Information, Use in Specific Populations, Pregnancy (8.1), Lactation (8.2), Females and Males of Reproductive Potential (8.3).]
There are no dietary restrictions for patients taking temozolomide capsules. Temozolomide capsules may affect testicular function and may cause birth defects. Advise male patients to exercise adequate birth control measures. Advise female patients to avoid becoming pregnant while receiving this drug. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 6 months after the last dose. Advise males of reproductive potential to use condoms during treatment and for at least 3 months after the last dose. Advise male patients not to donate semen during treatment with temozolomide capsules and for at least 3 months after the final dose. It is not known whether temozolomide is excreted into breast milk. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed while taking temozolomide capsules and for at least 1 week after the last dose.
What are the side effects of temozolomide capsules? [See Full Prescribing Information, Adverse Reactions (6).]
Alopecia, fatigue, nausea, and vomiting are the most common side effects associated with temozolomide capsules. Noncumulative myelosuppression is the dose-limiting toxicity. Patients should be evaluated periodically by their physician to monitor blood counts.
Other commonly reported side effects reported by patients taking temozolomide capsules are headache, constipation, anorexia, and convulsions.
How are temozolomide capsules supplied? [See Full Prescribing Information, How Supplied/Storage and Handling (16).]
Temozolomide Capsules, USP are available in 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, and 250 mg strengths. The capsules contain a white capsule body with a color cap, and the colors vary based on the dosage strength.
| Temozolomide Capsule, USP Strength | Color |
| 5 mg | Green Cap |
| 20 mg | Yellow Cap |
| 100 mg | Pink Cap |
| 140 mg | Blue Cap |
| 180 mg | Swedish Orange Cap |
| 250 mg | White Cap |
The 5 mg, 20 mg, 100 mg, 140 mg, 180 mg capsule strengths are available in 5 count, 14 count and 20 count packages. The 250-mg capsule strength is available in 5 count and 20 count packages.
How is temozolomide capsule dispensed?
Dispense each strength of temozolomide capsules in its original package (one strength per one container). Follow the instructions below:
Based on the dose prescribed, determine the number of each strength of temozolomide capsules needed for the full 42- or 5-day cycle as prescribed by the physician. For example, in a 5-day cycle, 275 mg/day would be dispensed as five 250 mg capsules, five 20 mg capsules and five 5 mg capsules. Label each container with the appropriate number of capsules to be taken each day. Dispense to the patient, making sure each container lists the strength (mg) per capsule and that he or she understands to take the appropriate number of capsules of temozolomide from each package to equal the total daily dose prescribed by the physician.
How can temozolomide capsules be ordered?
Temozolomide capsules can be ordered from your wholesaler. It is important to understand if temozolomide capsules are being used as part of a 42-day regimen or as part of a 5-day course. Remember to order enough temozolomide capsules for the appropriate cycle.
For example:
- a 5-day course of 360 mg/day would require the following to be ordered: two 5-count packages of 180 mg capsules.
- a 42-day course of 140 mg/day would require the following to be ordered: three 14-count packages of 140 mg capsules.
Temozolomide Capsules, USP 5 mg:
5 count: NDC 16571-816-51
14 count: NDC 16571-816-41
20 count: NDC 16571-816-02
Temozolomide Capsules, USP 20 mg:
5 count: NDC 16571-817-51
14 count: NDC 16571-817-41
20 count: NDC 16571-817-02
Temozolomide Capsules, USP 100 mg:
5 count: NDC 16571-818-51
14 count: NDC 16571-818-41
20 count: NDC 16571-818-02
Temozolomide Capsules, USP 140 mg:
5 count: NDC 16571-819-51
14 count: NDC 16571-819-41
20 count: NDC 16571-819-02
Temozolomide Capsules, USP 180 mg:
5 count: NDC 16571-820-51
14 count: NDC 16571-820-41
20 count: NDC 16571-820-02
Temozolomide Capsules, USP 250 mg:
5 count: NDC 16571-821-51
20 count: NDC 16571-821-02
References:
“OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Code No.: TS/DRUGS/24/2015
Revised: 03/2024
PLR82120-02
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Temozolomide Capsules, USP
5 mg - 5's container

Temozolomide Capsules, USP
20 mg - 5's container

Temozolomide Capsules, USP
100 mg - 5's container

Temozolomide Capsules, USP
140 mg - 5's container

Temozolomide Capsules, USP
180 mg - 5's container

Temozolomide Capsules, USP
250 mg - 5's container

| TEMOZOLOMIDE
temozolomide capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rising Pharma Holdings, Inc. (116880195) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eugia Pharma Specialities Limited | 650498244 | ANALYSIS(16571-816, 16571-817, 16571-818, 16571-819, 16571-820, 16571-821) , MANUFACTURE(16571-816, 16571-817, 16571-818, 16571-819, 16571-820, 16571-821) , PACK(16571-816, 16571-817, 16571-818, 16571-819, 16571-820, 16571-821) | |
More about temozolomide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (10)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: alkylating agents
- En español