Oxygen Nitrogen Mixture Prescribing Information
Package insert / product label
Dosage form: gas
Drug class: Medical gas
Medically reviewed by Drugs.com. Last updated on Nov 19, 2023.
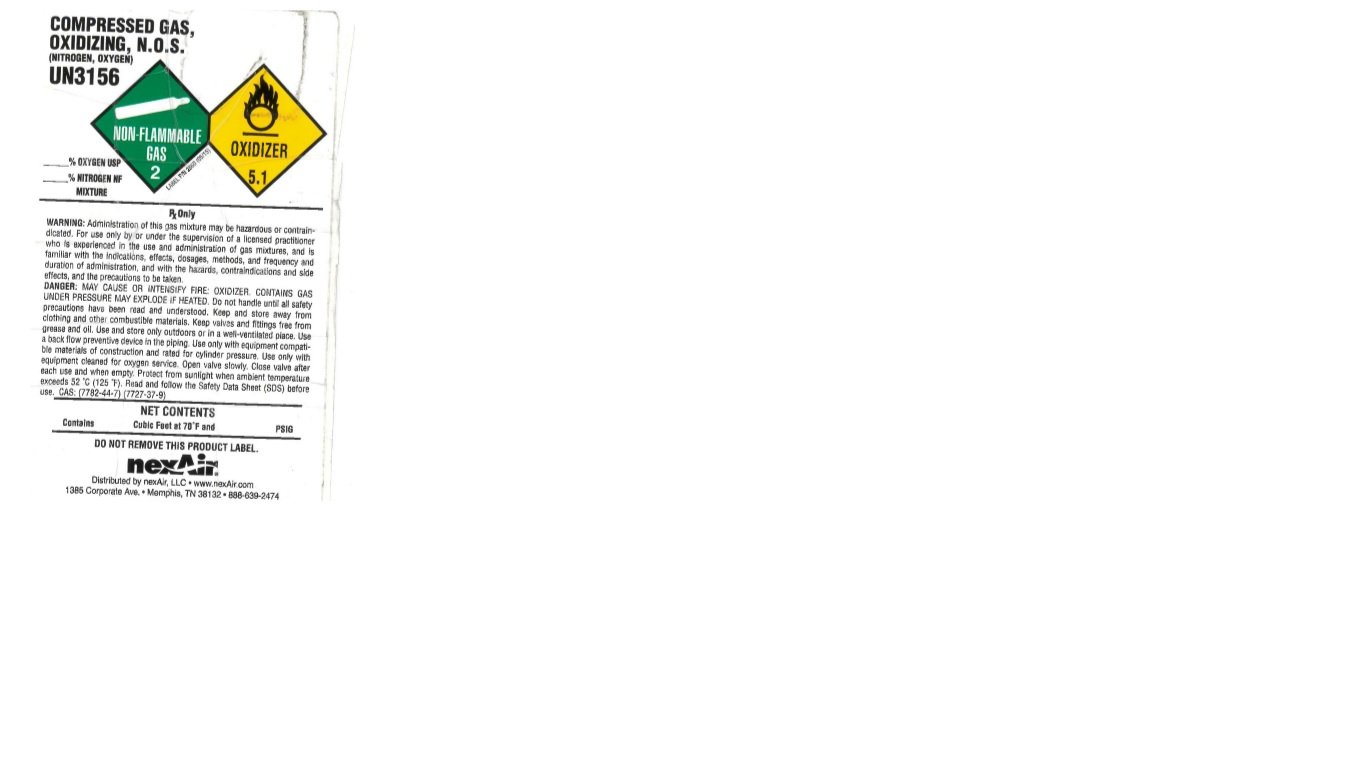
COMPRESSED GAS, OXIDIZING, N.O.S. (NITROGEN, OXYGEN)
UN3156
% OXYGEN USP
% NITROGEN NF MIXTURE
RX ONLY
WARNING: Administration of this gas mixture may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of gas mixtures, and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects, and the precautions to be taken.
DANGER: MAY CAUSE OR INTENSIFY FIRE: OXIDIZER. CONTAINS GAS UNDER PRESSURE MAY EXPLODE IF HEATED. Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. Use a back flow preventive device in the piping. Use only equipment compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52 C (125 F). Read and follow the Safety Data Sheet (SDS) before use. CAS: (7782-44-7) (7727-37-9)
NET CONTENTS
Contains Cubic Feet at 70 F and PSIG
DO NOT REMOVE THIS PRODUCT LABEL.
nexAir
Distributed by nexAir, LLC www.nexair.com
1385 Corporate Ave. Memphis, TN 38132 888-639-2474
| 40% OXYGEN/NITROGEN MIX
40% oxygen/nitrogen mix gas |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - NexAir LLC (927024422) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NEXAIR, LLC | 010263301 | manufacture(12213-200) | |
