Lidotor Cream Prescribing Information
Package insert / product label
Generic name: lidocaine and prilocaine
Dosage form: cream, kit
Drug class: Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Jan 15, 2023.
On This Page
Lidotor Cream Description
Lidocaine and Prilocaine Cream USP, 2.5%/2.5% is an emulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine in a ratio of 1:1 by weight. This eutectic mixture has a melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather than as crystals. It is packaged in 5 gram and 30 gram tubes. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol: water partition ratio of 43 at pH 7.4, and has the following structure:
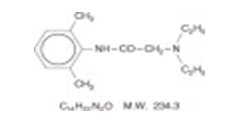
| C 14H 22N 2O | M.W. 234.3 | |
Prilocaine is chemically designated as propanamide, N-(2-methylphenyl)-2-(propylamino), has an octanol: water partition ratio of 25 at pH 7.4, and has the following structure:
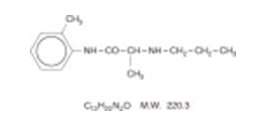
| C 13H 20N 2O | M.W. 220.3 | |
Each gram of lidocaine 2.5% and prilocaine cream, 2.5% cream contains lidocaine 25 mg, prilocaine 25 mg, carboxypolymethylene (as a thickening agent), polyoxyethylene fatty acid esters (as emulsifiers), purified water to 1 gram, and sodium hydroxide to adjust pH (pH range 9.0-9.4). Lidocaine 2.5% and prilocaine 2.5% cream contains no preservative, however it passes the USP antimicrobial effectiveness test due to the pH. The specific gravity of lidocaine 2.5% and prilocaine 2.5% cream is 1.00.
Lidotor Cream - Clinical Pharmacology
Mechanism of Action
Lidocaine 2.5% and prilocaine 2.5% cream, applied to intact skin under occlusive dressing, provides dermal analgesia by the release of lidocaine and prilocaine from the cream into the epidermal and dermal layers of the skin and by the accumulation of lidocaine and prilocaine in the vicinity of dermal pain receptors and nerve endings. Lidocaine and prilocaine are amide-type local anesthetic agents. Both lidocaine and prilocaine stabilize neuronal membranes by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
The onset, depth and duration of dermal analgesia on intact skin provided by lidocaine 2.5% and prilocaine 2.5% cream depend primarily on the duration of application. To provide sufficient analgesia for clinical procedures such as intravenous catheter placement and venipuncture, lidocaine 2.5% and prilocaine 2.5% cream should be applied under an occlusive dressing for at least 1 hour. To provide dermal analgesia for clinical procedures such as split skin graft harvesting, lidocaine 2.5% and prilocaine 2.5% cream should be applied under occlusive dressing for at least 2 hours. Satisfactory dermal analgesia is achieved 1 hour after application, reaches maximum at 2 to 3 hours, and persists for 1 to 2 hours after removal. Absorption from the genital mucosa is more rapid and onset time is shorter (5 to 10 minutes) than after application to intact skin. After a 5 to 10 minute application of lidocaine 2.5% and prilocaine 2.5% cream to female genital mucosa, the average duration of effective analgesia to an argon laser stimulus (which produced a sharp, pricking pain) was 15 to 20 minutes (individual variations in the range of 5 to 45 minutes).
Dermal application of lidocaine 2.5% and prilocaine 2.5% cream may cause a transient, local blanching followed by a transient, local redness or erythema.
Pharmacokinetics
Lidocaine 2.5% and prilocaine 2.5% cream is a eutectic mixture of lidocaine 2.5% and prilocaine 2.5% formulated as an oil in water emulsion. In this eutectic mixture, both anesthetics are liquid at room temperature (see DESCRIPTION) and the penetration and subsequent systemic absorption of both prilocaine and lidocaine are enhanced over that which would be seen if each component in crystalline form was applied separately as a 2.5% topical cream.
Absorption
Absorption: The amount of lidocaine and prilocaine systemically absorbed from lidocaine 2.5% and prilocaine 2.5% cream is directly related to both the duration of application and to the area over which it is applied. In two pharmacokinetic studies, 60 g of lidocaine 2.5% and prilocaine 2.5% cream (1.5 g lidocaine and 1.5 g prilocaine) was applied to 400 cm 2 of intact skin on the lateral thigh and then covered by an occlusive dressing. The subjects were then randomized such that one-half of the subjects had the occlusive dressing and residual cream removed after 3 hours, while the remainder left the dressing in place for 24 hours. The results from these studies are summarized below.
| Lidocaine 2.5% and Prilocaine 2.5% Cream (g) | Area (cm 2) | Time
on (hrs) | Drug Content (mg) | Absorbed (mg) | C max (µg/mL) | T max (hr) |
|---|---|---|---|---|---|---|
|
60 |
400 |
3 |
lidocaine 1500 |
54 |
0.12 |
4 |
|
prilocaine 1500 |
92 |
0.07 |
4 |
|||
|
60 |
400 |
24* |
lidocaine 1500 |
243 |
0.28 |
10 |
|
prilocaine 1500 |
503 |
0.14 |
10 |
When 60 g of lidocaine 2.5% and prilocaine 2.5% cream was applied over 400 cm 2 for 24 hours, peak blood levels of lidocaine are approximately 1/20 the systemic toxic level. Likewise, the maximum prilocaine level is about 1/36 the toxic level. In a pharmacokinetic study, lidocaine 2.5% and prilocaine 2.5% cream was applied to penile skin in 20 adult male patients in doses ranging from 0.5 g to 3.3 g for 15 minutes. Plasma concentrations of lidocaine and prilocaine following lidocaine 2.5% and prilocaine 2.5% cream application in this study were consistently low (2.5-16 ng/mL for lidocaine and 2.5-7 ng/mL for prilocaine). The application of lidocaine 2.5% and prilocaine 2.5% cream to broken or inflamed skin, or to 2,000 cm 2 or more of skin where more of both anesthetics are absorbed, could result in higher plasma levels that could, in susceptible individuals, produce a systemic pharmacologic response.
The absorption of lidocaine 2.5% and prilocaine 2.5% cream applied to genital mucous membranes was studied in two open-label clinical trials. Twenty-nine patients received 10 g of lidocaine 2.5% and prilocaine 2.5% cream applied for 10 to 60 minutes in the vaginal fornices. Plasma concentrations of lidocaine and prilocaine following lidocaine 2.5% and prilocaine 2.5% cream application in these studies ranged from 148 to 641 ng/mL for lidocaine and 40 to 346 ng/mL for prilocaine and time to reach maximum concentration (t max) ranged from 21 to 125 minutes for lidocaine and from 21 to 95 minutes for prilocaine. These levels are well below the concentrations anticipated to give rise to systemic toxicity (approximately 5000 ng/mL for lidocaine and prilocaine).
Distribution: When each drug is administered intravenously, the steady-state volume of distribution is 1.1 to 2.1 L/kg (mean 1.5, ±0.3 SD, n=13) for lidocaine and is 0.7 to 4.4 L/kg (mean 2.6, ± 1.3 SD, n=13) for prilocaine. The larger distribution volume for prilocaine produces the lower plasma concentrations of prilocaine observed when equal amounts of prilocaine and lidocaine are administered. At concentrations produced by application of lidocaine 2.5% and prilocaine 2.5% cream, lidocaine is approximately 70% bound to plasma proteins, primarily alpha-1-acid glycoprotein. At much higher plasma concentrations (1 to 4 µg/mL of free base) the plasma protein binding of lidocaine is concentration dependent. Prilocaine is 55% bound to plasma proteins. Both lidocaine and prilocaine cross the placental and blood brain barrier, presumably by passive diffusion.
Metabolism: It is not known if lidocaine or prilocaine are metabolized in the skin. Lidocaine is metabolized rapidly by the liver to a number of metabolites including monoethylglycinexylidide (MEGX) and glycinexylidide (GX), both of which have pharmacologic activity similar to, but less potent than that of lidocaine. The metabolite, 2,6-xylidine, has unknown pharmacologic activity. Following intravenous administration, MEGX and GX concentrations in serum range from 11 to 36% and from 5 to 11% of lidocaine concentrations, respectively. Prilocaine is metabolized in both the liver and kidneys by amidases to various metabolites including ortho-toluidine and N-n-propylalanine. It is not metabolized by plasma esterases. The ortho-toluidine metabolite has been shown to be carcinogenic in several animal models (see Carcinogenesis subsection of PRECAUTIONS). In addition, ortho-toluidine can produce methemoglobinemia following systemic doses of prilocaine approximating 8 mg/kg (see ADVERSE REACTIONS). Very young patients, patients with glucose-6-phosphate dehydrogenase deficiencies and patients taking oxidizing drugs such as antimalarials and sulfonamides are more susceptible to methemoglobinemia (see Methemoglobinemia subsection of PRECAUTIONS).
Elimination - The terminal elimination half-life of lidocaine from the plasma following IV administration is approximately 65 to 150 minutes (mean 110, ±24 SD, n=13). More than 98% of an absorbed dose of lidocaine can be recovered in the urine as metabolites or parent drug. The systemic clearance is 10 to 20 mL/min/kg (mean 13, ±3 SD, n=13). The elimination half-life of prilocaine is approximately 10 to 150 minutes (mean 70, ±48 SD, n=13). The systemic clearance is 18 to 64 mL/min/kg (mean 38, ±15 SD, n=13). During intravenous studies, the elimination half-life of lidocaine was statistically significantly longer in elderly patients (2.5 hours) than in younger patients (1.5 hours). No studies are available on the intravenous pharmacokinetics of prilocaine in elderly patients.
Pediatrics - Some pharmacokinetic (PK) data are available in infants (1 month to <2 years old) and children (2 to <12 years old). One PK study was conducted in 9 full-term neonates (mean age: 7 days and mean gestational age: 38.8 weeks). The study results show that neonates had comparable plasma lidocaine and prilocaine concentrations and blood methemoglobin concentrations as those found in previous pediatric PK studies and clinical trials. There was a tendency towards an increase in methemoglobin formation. However, due to assay limitations and very little amount of blood that could be collected from neonates, large variations in the above reported concentrations were found.
Special Populations - No specific PK studies were conducted. The half-life may be increased in cardiac or hepatic dysfunction. Prilocaine’s half-life also may be increased in hepatic or renal dysfunction since both of these organs are involved in prilocaine metabolism.
Distribution
When each drug is administered intravenously, the steady-state volume of distribution is 1.1 to 2.1 L/kg (mean 1.5, ±0.3 SD, n=13) for lidocaine and is 0.7 to 4.4 L/kg (mean 2.6, ± 1.3 SD, n=13) for prilocaine. The larger distribution volume for prilocaine produces the lower plasma concentrations of prilocaine observed when equal amounts of prilocaine and lidocaine are administered. At concentrations produced by application of lidocaine 2.5% and prilocaine 2.5% cream, lidocaine is approximately 70% bound to plasma proteins, primarily alpha-1-acid glycoprotein. At much higher plasma concentrations (1 to 4 µg/mL of free base) the plasma protein binding of lidocaine is concentration dependent. Prilocaine is 55% bound to plasma proteins. Both lidocaine and prilocaine cross the placental and blood brain barrier, presumably by passive diffusion.
Metabolism
It is not known if lidocaine or prilocaine are metabolized in the skin. Lidocaine is metabolized rapidly by the liver to a number of metabolites including monoethylglycinexylidide (MEGX) and glycinexylidide (GX), both of which have pharmacologic activity similar to, but less potent than that of lidocaine. The metabolite, 2,6-xylidine, has unknown pharmacologic activity. Following intravenous administration, MEGX and GX concentrations in serum range from 11 to 36% and from 5 to 11% of lidocaine concentrations, respectively. Prilocaine is metabolized in both the liver and kidneys by amidases to various metabolites including ortho-toluidine and N-n-propylalanine. It is not metabolized by plasma esterases. The ortho-toluidine metabolite has been shown to be carcinogenic in several animal models (see Carcinogenesis subsection of PRECAUTIONS). In addition, ortho-toluidine can produce methemoglobinemia following systemic doses of prilocaine approximating 8 mg/kg (see ADVERSE REACTIONS). Very young patients, patients with glucose-6-phosphate dehydrogenase deficiencies and patients taking oxidizing drugs such as antimalarials and sulfonamides are more susceptible to methemoglobinemia (see Methemoglobinemia subsection of PRECAUTIONS).
Elimination
The terminal elimination half-life of lidocaine from the plasma following IV administration is approximately 65 to 150 minutes (mean 110, ±24 SD, n=13). More than 98% of an absorbed dose of lidocaine can be recovered in the urine as metabolites or parent drug. The systemic clearance is 10 to 20 mL/min/kg (mean 13, ±3 SD, n=13). The elimination half-life of prilocaine is approximately 10 to 150 minutes (mean 70, ±48 SD, n=13). The systemic clearance is 18 to 64 mL/min/kg (mean 38, ±15 SD, n=13). During intravenous studies, the elimination half-life of lidocaine was statistically significantly longer in elderly patients (2.5 hours) than in younger patients (1.5 hours). No studies are available on the intravenous pharmacokinetics of prilocaine in elderly patients.
Pediatrics
Some pharmacokinetic (PK) data are available in infants (1 month to <2 years old) and children (2 to <12 years old). One PK study was conducted in 9 full-term neonates (mean age: 7 days and mean gestational age: 38.8 weeks). The study results show that neonates haad comparable plasma lidocaine and prilocaine concentrations and blood methemoglobin concentrations as those found in previous pediatric PK studies and clinical trials. There was a tendency towards an increase in methemoglobin formation. However, due to assay limitations and very little amount of blood that could be collected from neonates, large variations in the above reported concentrations were found.
CLINICAL STUDIES
Lidocaine 2.5% and prilocaine 2.5% cream application in adults prior to IV cannulation or venipuncture was studied in 200 patients in four clinical studies in Europe. Application for at least 1 hour provided significantly more dermal analgesia than placebo cream or ethyl chloride. Lidocaine 2.5% and prilocaine 2.5% cream was comparable to subcutaneous lidocaine, but was less efficacious than intradermal lidocaine. Most patients found lidocaine 2.5% and prilocaine 2.5% cream treatment preferable to lidocaine infiltration or ethyl chloride spray.
Lidocaine 2.5% and prilocaine 2.5% cream was compared with 0.5% lidocaine infiltration prior to skin graft harvesting in one open label study in 80 adult patients in England. Application of lidocaine 2.5% and prilocaine 2.5% cream for 2 to 5 hours provided dermal analgesia comparable to lidocaine infiltration.
Lidocaine 2.5% and prilocaine 2.5% cream application in children was studied in seven non-US studies (320 patients) and one US study (100 patients). In controlled studies, application of lidocaine 2.5% and prilocaine 2.5% cream for at least 1 hour with or without presurgical medication prior to needle insertion provided significantly more pain reduction than placebo. In children under the age of seven years, lidocaine 2.5% and prilocaine 2.5% cream was less effective than in older children or adults.
Lidocaine 2.5% and prilocaine 2.5% cream was compared with placebo in the laser treatment of facial port-wine stains in 72 pediatric patients (ages 5−16). Lidocaine 2.5% and prilocaine 2.5% cream was effective in providing pain relief during laser treatment.
Lidocaine 2.5% and prilocaine 2.5% cream alone was compared with lidocaine 2.5% and prilocaine 2.5% cream followed by lidocaine infiltration and lidocaine infiltration alone prior to cryotherapy for the removal of male genital warts. The data from 121 patients demonstrated that lidocaine 2.5% and prilocaine 2.5% cream was not effective as a sole anesthetic agent in managing the pain from the surgical procedure. The administration of lidocaine 2.5% and prilocaine 2.5% cream prior to lidocaine infiltration provided significant relief of discomfort associated with local anesthetic infiltration and thus was effective in the overall reduction of pain from the procedure only when used in conjunction with local anesthetic infiltration of lidocaine.
Lidocaine 2.5% and prilocaine 2.5% cream was studied in 105 full term neonates (gestational age: 37 weeks) for blood drawing and circumcision procedures. When considering the use of lidocaine 2.5% and prilocaine 2.5% cream in neonates, the primary concerns are the systemic absorption of the active ingredients and the subsequent formation of methemoglobin. In clinical studies performed in neonates, the plasma levels of lidocaine, prilocaine, and methemoglobin were not reported in a range expected to cause clinical symptoms.
Local dermal effects associated with lidocaine 2.5% and prilocaine 2.5% cream application in these studies on intact skin included paleness, redness and edema and were transient in nature (see ADVERSE REACTIONS).
The application of lidocaine 2.5% and prilocaine 2.5% cream on genital mucous membranes for minor, superficial surgical procedures (eg, removal of condylomata acuminata) was studied in 80 patients in a placebo-controlled clinical trial (60 patients received lidocaine 2.5% and prilocaine 2.5% cream and 20 patients received placebo). Lidocaine 2.5% and prilocaine 2.5% cream (5 to 10 g) applied between 1 and 75 minutes before surgery, with a median time of 15 minutes, provided effective local anesthesia for minor superficial surgical procedures. The greatest extent of analgesia, as measured by VAS scores, was attained after 5 to 15 minutes’ application. The application of lidocaine 2.5% and prilocaine 2.5% cream to genital mucous membranes as pretreatment for local anesthetic infiltration was studied in a double-blind, placebo-controlled study in 44 female patients (21 patients received lidocaine 2.5% and prilocaine 2.5% cream and 23 patients received placebo) scheduled for infiltration prior to a surgical procedure of the external vulva or genital mucosa. Lidocaine 2.5% and prilocaine 2.5% cream applied to the genital mucous membranes for 5 to 10 minutes resulted in adequate topical anesthesia for local anesthetic injection.
Individualization of Dose
The dose of lidocaine 2.5% and prilocaine 2.5% cream that provides effective analgesia depends on the duration of the application over the treated area.
All pharmacokinetic and clinical studies employed a thick layer of lidocaine 2.5% and prilocaine 2.5% cream (1−2 g/10 cm 2). The duration of application prior to venipuncture was 1 hour. The duration of application prior to taking split thickness skin grafts was 2 hours. A thinner application has not been studied and may result in less complete analgesia or a shorter duration of adequate analgesia.
The systemic absorption of lidocaine and prilocaine is a side effect of the desired local effect. The amount of drug absorbed depends on surface area and duration of application. The systemic blood levels depend on the amount absorbed and patient size (weight) and the rate of systemic drug elimination. Long duration of application, large treatment area, small patients, or impaired elimination may result in high blood levels. The systemic blood levels are typically a small fraction (1/20 to 1/36) of the blood levels that produce toxicity. Table 2 below gives maximum recommended doses, application areas, and application times for infants and children.
| For Infants and Children Based on Application to Intact Skin | |||
|---|---|---|---|
| Age and Body Weight Requirements | Maximum Total Dose of lidocaine 2.5% and prilocaine 2.5% cream | Maximum Application Area** | Maximum Application Time |
|
0 up to 3 months or < 5 kg |
1 g |
10 cm 2 |
1 hour |
|
3 up to 12 months and > 5 kg |
2 g |
20 cm 2 |
4 hours |
|
1 to 6 years and > 10 kg |
10 g |
100 cm 2 |
4 hours |
|
7 to 12 years and > 20 kg |
20 g |
200 cm 2 |
4 hours |
|
Please note: If a patient greater than 3 months old does not meet the minimum weight requirement, the maximum total dose of lidocaine 2.5% and prilocaine 2.5% cream should be restricted to that which corresponds to the patient’s weight |
|||
An I.V. antiarrhythmic dose of lidocaine is 1 mg/kg (70 mg/70 kg) and gives a blood level of about 1 µg/mL. Toxicity would be expected at blood levels above 5 µg/mL. Smaller areas of treatment are recommended in a debilitated patient, a small child or a patient with impaired elimination. Decreasing the duration of application is likely to decrease the analgesic effect.
Indications and Usage for Lidotor Cream
Lidocaine 2.5% and prilocaine 2.5% cream (a eutectic mixture of lidocaine 2.5% and prilocaine 2.5%) is indicated as a topical anesthetic for use on:
- normal intact skin for local analgesia.
- Genital mucous membranes for superficial minor surgery and as pretreatment for infiltration anesthesia.
Lidocaine 2.5% and prilocaine 2.5% cream is not recommended in any clinical situation when penetration or migration beyond the tympanic membrane into the middle ear is possible because of the ototoxic effects observed in animal studies (see WARNINGS).
Contraindications
Lidocaine 2.5% and prilocaine 2.5% cream (lidocaine 2.5% and prilocaine 2.5%) is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type or to any other component of the product.
Warnings
Application of lidocaine 2.5% and prilocaine 2.5% cream to larger areas or for longer times than those recommended could result in sufficient absorption of lidocaine and prilocaine resulting in serious adverse effects (see Individualization of Dose).
Patients treated with class III anti-arrhythmic drugs (eg, amiodarone, bretylium, sotalol, dofetilide) should be under close surveillance and ECG monitoring considered, because cardiac effects may be additive.
Studies in laboratory animals (guinea pigs) have shown that lidocaine 2.5% and prilocaine 2.5% cream has an ototoxic effect when instilled into the middle ear. In these same studies, animals exposed to lidocaine 2.5% and prilocaine 2.5% cream only in the external auditory canal, showed no abnormality. lidocaine 2.5% and prilocaine 2.5% cream should not be used in any clinical situation when its penetration or migration beyond the tympanic membrane into the middle ear is possible.
Methemoglobinemia
Lidocaine 2.5% and prilocaine 2.5% cream should not be used in those rare patients with congenital or idiopathic methemoglobinemia and in infants under the age of twelve months who are receiving treatment with methemoglobin-inducing agents.
Very young patients or patients with glucose-6-phosphate dehydrogenase deficiencies are more susceptible to methemoglobinemia.
Patients taking drugs associated with drug-induced methemoglobinemia such as sulfonamides, acetaminophen, acetanilid, aniline dyes, benzocaine, chloroquine, dapsone, naphthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, paraaminosalicylic acid, phenacetin, phenobarbital, phenytoin, primaquine, quinine, are also at greater risk for developing methemoglobinemia.
There have been reports of significant methemoglobinemia (20-30%) in infants and children following excessive applications of lidocaine 2.5% and prilocaine 2.5% cream. These cases involved the use of large doses, larger than recommended areas of application, or infants under the age of 3 months who did not have fully mature enzyme systems. In addition, a few of these cases involved the concomitant administration of methemoglobin-inducing agents. Most patients recovered spontaneously after removal of the cream. Treatment with IV methylene blue may be effective if required.
Physicians are cautioned to make sure that parents or other caregivers understand the need for careful application of lidocaine 2.5% and prilocaine 2.5% cream, to ensure that the doses and areas of application recommended in Table 2 are not exceeded (especially in children under the age of 3 months) and to limit the period of application to the minimum required to achieve the desired anesthesia.
Neonates and infants up to 3 months of age should be monitored for Met-Hb levels before, during, and after the application of lidocaine 2.5% and prilocaine 2.5% cream, provided the test results can be obtained quickly.
Precautions
General
Repeated doses of lidocaine 2.5% and prilocaine 2.5% cream may increase blood levels of lidocaine and prilocaine. lidocaine 2.5% and prilocaine 2.5% cream should be used with caution in patients who may be more sensitive to the systemic effects of lidocaine and prilocaine including acutely ill, debilitated, or elderly patients.
Lidocaine 2.5% and prilocaine 2.5% cream should not be applied to open wounds.
Care should be taken not to allow lidocaine 2.5% and prilocaine 2.5% cream to come in contact with the eye because animal studies have demonstrated severe eye irritation. Also the loss of protective reflexes can permit corneal irritation and potential abrasion. Absorption of lidocaine 2.5% and prilocaine 2.5% cream in conjunctival tissues has not been determined. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
Patients allergic to paraaminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine and/or prilocaine; however, lidocaine 2.5% and prilocaine 2.5% cream should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain.
Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations of lidocaine and prilocaine.
Lidocaine and prilocaine have been shown to inhibit viral and bacterial growth. The effect of lidocaine 2.5% and prilocaine 2.5% cream on intradermal injections of live vaccines has not been determined.
Information for Patients
When lidocaine 2.5% and prilocaine 2.5% cream is used, the patient should be aware that the production of dermal analgesia may be accompanied by the block of all sensations in the treated skin. For this reason, the patient should avoid inadvertent trauma to the treated area by scratching, rubbing, or exposure to extreme hot or cold temperatures until complete sensation has returned.
Lidocaine 2.5% and prilocaine 2.5% cream should not be applied near the eyes or on open wounds.
Drug Interactions
Lidocaine 2.5% and prilocaine 2.5% cream should be used with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic.
Prilocaine may contribute to the formation of methemoglobin in patients treated with other drugs known to cause this condition (see Methemoglobinemia subsection of WARNINGS).
Specific intreaction studies with lidocaine/prilocaine and class III anti-arrhythmic drugs (eg, amiodarone, bretylium, sotalol, dofetilide) have not been performed, but caution is advised ( see WARNINGS).
Should lidocaine 2.5% and prilocaine 2.5% cream be used concomitantly with other products containing lidocaine and/or prilocaine, cumulative doses from all formulations must be considered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals designed to evaluate the carcinogenic potential of lidocaine and prilocaine have not been conducted.
Metabolites of prilocaine have been shown to be carcinogenic in laboratory animals. In the animal studies reported below, doses or blood levels are compared with the Single Dermal Administration (SDA) of 60 g of lidocaine 2.5% and prilocaine 2.5% cream to 400 cm2 for 3 hours to a small person (50 kg). The typical application of lidocaine 2.5% and prilocaine 2.5% cream for one or two treatments for venipuncture sites (2.5 or 5 g) would be 1/24 or 1/12 of that dose in an adult or about the same mg/kg dose in an infant.
Chronic oral toxicity studies of ortho-toluidine, a metabolite of prilocaine, in mice (450 to 7,200 mg/m2; 60 to 960 times SDA) and rats (900 to 4,800 mg/m2; 60 to 320 times SDA) have shown that ortho-toluidine is a carcinogen in both species. The tumors included hepatocarcinomas/adenomas in female mice, multiple occurrences of hemangiosarcomas/hemangiomas in both sexes of mice, sarcomas of multiple organs, transitional-cell carcinomas/papillomas of urinary bladder in both sexes of rats, subcutaneous fibromas/fibrosarcomas and mesotheliomas in male rats, and mammary gland fibroadenomas/adenomas in female rats. The lowest dose tested (450 mg/m2 in mice, 900 mg/m2 in rats, 60 times SDA) was carcinogenic in both species. Thus the no-effect dose must be less than 60 times SDA. The animal studies were conducted at 150 to 2,400 mg/kg in mice and at 150 to 800 mg/kg in rats. The dosages have been converted to mg/m2 for the SDA calculations above.
Mutagenesis
The mutagenic potential of lidocaine HCL has been tested in a bacterial reverse (Ames) assay in Salmonella, an invitro chromosomal aberration assay using human lymphocytes and in an in vivo micronucleus test in mice. There was no indication of mutagenicity or structural damage to chromosomes in these tests.
Ortho-toluidine, a metabolite of prilocaine, at a concentration of 0.5 µg/mL, was genotoxic in Escherichia coli DNA repair and phage-induction assays. Urine concentrates from rats treated with ortho-toluidine (300 mg/kg orally; 300 times SDA) were mutagenic when examined in Salmonella typhimurium in the presence of metabolic activation. Several other tests on ortho-toluidine, including reverse mutations in five different Salmonella typhimurium strains in the presence or absence of metabolic activation and a study to detect single strand breaks in DNA of V79 Chinese hamster cells, were negative.
Use in Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies with lidocaine have been performed in rats and have revealed no evidence of harm to the fetus (30 mg/kg subcutaneously; 22 times SDA). Reproduction studies with prilocaine have been performed in rats and have revealed no evidence of impaired fertility or harm to the fetus (300 mg/kg intramuscularly; 188 times SDA). There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, lidocaine and prilocaine cream, 2.5%/2.5% should be used during pregnancy only if clearly needed.
Reproduction studies have been performed in rats receiving subcutaneous administration of an aqueous mixture containing lidocaine HCl and prilocaine HCl at 1:1 (w/w). At 40 mg/kg each, a dose equivalent to 29 times SDA lidocaine and 25 times SDA prilocaine, no teratogenic, embryotoxic or fetotoxic effects were observed.
Labor and Delivery
Neither lidocaine nor prilocaine are contraindicated in labor and delivery. Should lidocaine 2.5% and prilocaine 2.5% cream be used concomitantly with other products containing lidocaine and/or prilocaine, cumulative doses from all formulations must be considered.
Nursing Mothers
Lidocaine, and probably prilocaine, are excreted in human milk. Therefore, caution should be exercised when lidocaine 2.5% and prilocaine 2.5% cream is administered to a nursing mother since the milk: plasma ratio of lidocaine is 0.4 and is not determined for prilocaine.
Pediatric Use
Controlled studies of lidocaine 2.5% and prilocaine 2.5% cream in children under the age of seven years have shown less overall benefit than in older children or adults. These results illustrate the importance of emotional and psychological support of younger children undergoing medical or surgical procedures.
Lidocaine 2.5% and prilocaine 2.5% cream should be used with care in patients with conditions or therapy associated with methemoglobinemia (see Methemoglobinemia subsection of WARNINGS).
When using lidocaine 2.5% and prilocaine 2.5% cream in young children, especially infants under the age of 3 months, care must be taken to insure that the caregiver understands the need to limit the dose and area of application, and to prevent accidental ingestion (see DOSAGE AND ADMINISTRATION and Methemoglobinemia ).
In neonates (minimum gestation age; 37 weeks) and children weighing less than 20 kg, the area and duration of application should be limited (see TABLE 2 in Individualization of Dose).
Studies have not demonstrated the efficacy of lidocaine and prilocaine cream, 2.5%/2.5% for heel lancing in neonates.
Geriatric Use
Of the total number of patients in clinical studies of lidocaine 2.5% and prilocaine 2.5% cream, 180 were age 65 to 74 and 138 were 75 and over. No overall differences in safety or efficacy were observed between these patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Plasma levels of lidocaine and prilocaine in geriatric and non-geriatric patients following application of a thick layer of lidocaine 2.5% and prilocaine 2.5% cream are very low and well below potentially toxic levels. However, there are no sufficient data to evaluate quantitative differences in systemic plasma levels of lidocaine and prilocaine between geriatric and non-geriatric patients following application of lidocaine 2.5% and prilocaine 2.5% cream.
Consideration should be given for those elderly patients who have enhanced sensitivity to systemic absorption. (See PRECAUTIONS).
After intravenous dosing, the elimination half-life of lidocaine is significantly longer in elderly patients (2.5 hours) than in younger patients (1.5 hours). (See CLINICAL PHARMACOLOGY ).
Adverse Reactions/Side Effects
To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Localized Reactions
During or immediately after treatment with lidocaine 2.5% and prilocaine 2.5% cream on intact skin, the skin at the site of treatment may develop erythema or edema or may be the locus of abnormal sensation. Rare cases of discrete purpuric or petechial reactions at the application site have been reported. Rare cases of hyperpigmentation following the use of lidocaine 2.5% and prilocaine 2.5% cream have been reported. The relationship to lidocaine 2.5% and prilocaine 2.5% cream or the underlying procedure has not been established. In clinical studies on intact skin involving over 1,300 lidocaine 2.5% and prilocaine 2.5% cream-treated subjects, one or more such local reactions were noted in 56% of patients, and were generally mild and transient, resolving spontaneously within 1 or 2 hours. There were no serious reactions that were ascribed to lidocaine 2.5% and prilocaine 2.5% cream.
Two recent reports describe blistering on the foreskin in neonates about to undergo circumcision. Both neonates received 1.0 g of lidocaine 2.5% and prilocaine 2.5% cream.
In patients treated with lidocaine 2.5% and prilocaine 2.5% cream on intact skin, local effects observed in the trials included: paleness (pallor or blanching) 37%, redness (erythema) 30%, alterations in temperature sensations 7%, edema 6%, itching 2% and rash, less than 1%.
In clinical studies on genital mucous membranes involving 378 lidocaine 2.5% and prilocaine 2.5% cream-treated patients, one or more application site reactions, usually mild and transient, were noted in 41% of patients. The most common application site reactions were redness (21%), burning sensation (17%) and edema (10%).
Allergic Reactions
Allergic and anaphylactoid reactions associated with lidocaine or prilocaine can occur. They are characterized by urticaria, angioedema, bronchospasm, and shock. If they occur they should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
Systemic (Dose Related) Reactions
Systemic adverse reactions following appropriate use of lidocaine 2.5% and prilocaine 2.5% cream are unlikely due to the small dose absorbed (see Pharmacokinetics subsection of CLINICAL PHARMACOLOGY). Systemic adverse effects of lidocaine and/or prilocaine are similar in nature to those observed with other amide local anesthetic agents including CNS excitation and/or depression (light-headedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest). Excitatory CNS reactions may be brief or not occur at all, in which case the first manifestation may be drowsiness merging into unconsciousness. Cardiovascular manifestations may include bradycardia, hypotension and cardiovascular collapse leading to arrest.
Overdosage
Peak blood levels following a 60 g application to 400 cm 2 of intact skin for 3 hours are 0.05 to 0.16 µg/mL for lidocaine and 0.02 to 0.10 µg/mL for prilocaine. Toxic levels of lidocaine (>5 µg/mL) and/or prilocaine (>6 µg/mL) cause decreases in cardiac output, total peripheral resistance and mean arterial pressure. These changes may be attributable to direct depressant effects of these local anesthetic agents on the cardiovascular system. In the absence of massive topical overdose or oral ingestion, evaluation should include evaluation of other etiologies for the clinical effects or overdosage from other sources of lidocaine, prilocaine or other local anesthetics. Consult the package inserts for parenteral Xylocaine (lidocaine HCl) or Citanest (prilocaine HCl) for further information for the management of overdose.
Lidotor Cream Dosage and Administration
Adult Patients-Intact Skin
A thick layer of Lidocaine and Prilocaine Cream USP, 2.5%/2.5% is applied to intact skin and covered with an occlusive dressing (see
INSTRUCTIONS FOR APPLICATION).
Minor Dermal Procedures
For minor procedures such as intravenous cannulation and venipuncture, apply 2.5 grams of lidocaine 2.5% and prilocaine 2.5% cream (1/2 the 5 g tube) over 20 to 25 cm2 of skin surface for at least 1 hour. In controlled clinical trials using lidocaine 2.5% and prilocaine 2.5% cream, two sites were usually prepared in case there was a technical problem with cannulation or venipuncture at the first site.
Major Dermal Procedures
For more painful dermatological procedures involving a larger skin area such as split thickness skin graft harvesting, apply 2 grams of lidocaine 2.5% and prilocaine 2.5% cream per 10 cm2 of skin and allow to remain in contact with the skin for at least 2 hours.
Adult Male Genital Skin
s an adjunct prior to local anesthetic infiltration, apply a thick layer of lidocaine 2.5% and prilocaine 2.5% cream (1 g/10 cm2) to the skin surface for 15 minutes. Local anesthetic infiltration should be performed immediately after removal of lidocaine 2.5% and prilocaine 2.5% cream.
Dermal analgesia can be expected to increase for up to 3 hours under occlusive dressing and persist for 1 to 2 hours after removal of the cream. The amount of lidocaine and prilocaine absorbed during the period of application can be estimated from the information in Table 2 , † footnote, in Individualization of Dose.
Adult Female Patients-Genital Mucous Membranes
For minor procedures on the female external genitalia, such as removal of condylomata acuminate, as well as for use as pretreatment for anesthetic infiltration, apply a thick layer (5 to 10 grams) of Lidocaine and Prilocaine Cream USP, 2.5%/2.5% for 5 to 10 minutes.
Occlusion is not necessary for absorption, but may be helpful to keep the cream in place. Patients should be lying down during the Lidocaine and Prilocaine Cream USP, 2.5%/2.5% application, especially if no occlusion is used. The procedure or the local anesthetic infiltration should be performed immediately after the removal of Lidocaine and Prilocaine Cream USP, 2.5%/2.5%.
Pediatric Patients-Intact Skin
The following are the maximum recommended doses, application areas and application times for lidocaine 2.5% and prilocaine 2.5% cream based on a child’s age and weight:
| Age and Body Weight Requirements | Maximum Total Dose of Lidocaine and Prilocaine Cream USP, 2.5%/2.5% | Maximum Application Area | Maximum Application Time |
|---|---|---|---|
| 0 up to 3 months or < 5 kg | 1 g | 10cm 2 | 1 hour |
| 3 up to 12 months and >5 kg | 2 g | 20cm 2 | 4 hours |
| 1 to 6 years and > 10 kg | 10 g | 100cm 2 | 4 hours |
| 7 to 12 years and > 20 kg | 20 g | 200cm 2 | 4 hours |
Please note: If a patient greater than 3 months old does not meet the minimum weight requirement, the maximum total dose of Lidocaine and Prilocaine Cream USP, 2.5%/2.5% should be restricted to that which corresponds to the patient's weight (see INSTRUCTIONS FOR APPLICATION ).
Practitioners should carefully instruct caregivers to avoid application of excessive amounts of lidocaine 2.5% and prilocaine 2.5% cream (see PRECAUTIONS ).
When applying lidocaine 2.5% and prilocaine 2.5% cream to the skin of young children, care must be taken to maintain careful observation of the child to prevent accidental ingestion of lidocaine 2.5% and prilocaine 2.5% cream or the occlusive dressing. A secondary protective covering to prevent inadvertent disruption of the application site may be useful.
Lidocaine 2.5% and prilocaine 2.5% cream should not be used in neonates with a gestational age less than 37 weeks nor in infants under the age of 12 months who are receiving treatment with methemoglobin-inducing agents (see Methemoglobinemia subsection of WARNINGS).
When lidocaine 2.5% and prilocaine 2.5% cream (lidocaine 2.5% and prilocaine 2.5%) is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered (see Individualization of Dose). The amount absorbed in the case of lidocaine 2.5% and prilocaine 2.5% cream is determined by the area over which it is applied and the duration of application under occlusion (seeTable 2 , footnote, in Individualization of Dose).
Although the incidence of systemic adverse reactions with lidocaine 2.5% and prilocaine 2.5% cream is very low, caution should be exercised, particularly when applying it over large areas and leaving it on for longer than 2 hours. The incidence of systemic adverse reactions can be expected to be directly proportional to the area and time of exposure (see Individualization of Dose).
INSTRUCTIONS FOR APPLICATION
To measure 1 gram of Lidocaine and Prilocaine Cream USP, 2.5%/2.5%, the cream should be gently squeezed out of the tube as a narrow strip that is 1.5 inches (3.8 cm) long and 0.2 inches (5mm) wide. The strip of Lidocaine and Prilocaine Cream USP, 2.5%/2.5% should be contained within the lines of the diagram shown below.
| ≈ 1 g strip | |
|
1.5 × 0.2 inches | |
Use the number of strips that equals your dose, like the examples in the table below.
Dosing information
1 gram = 1 strip
2 grams = 2 strips
2.5 grams = 2.5 strips
For adult and pediatric patients, apply ONLY as prescribed by your physician.
If your child is below the age of 3 months or small for their age, please inform your doctor before applying Lidocaine and Prilocaine Cream USP, 2.5%/2.5%, which can be harmful, if applied over too much skin at one time in young children.
When applying Lidocaine and Prilocaine Cream USP, 2.5%/2.5% to the intact skin of young children, it is important that they be carefully observed by an adult in order to prevent the accidental ingestion of or eye contact with Lidocaine and Prilocaine Cream USP, 2.5%/2.5%.
Lidocaine and Prilocaine Cream USP, 2.5%/2.5% must be applied to intact skin at least 1 hour before the start of a routine procedure and for 2 hours before the start of a painful procedure. A protective covering of the cream is not necessary for absorption but may be helpful to keep the cream in place.
If using a protective covering, your doctor will remove it, wipe off the Lidocaine and Prilocaine Cream USP, 2.5%/2.5%, and clean the entire area with an antiseptic solution before the procedure. The duration of effective skin anesthesia will be at least 1 hour after removal of the protective covering.
PRECAUTIONS
- Do not apply near eyes or on open wounds.
- Keep out of the reach of children
- If your child becomes very dizzy, excessively sleepy, or develops duskiness of the face or lips after applying Lidocaine and Prilocaine Cream USP, 2.5%/2.5%, remove the cream and contact the child's physician at once.
How is Lidotor Cream supplied
Lidocaine 2.5% and Prilocaine 2.5% Cream is available as the following:
| NDC No. | Strength | Size |
|---|---|---|
| NDC 50383-667-30 | 30 gram tube | (in a child-resistant tube) |
Frame Style Transparent Dressing (5 Count)
INSTRUCTIONS FOR USE DESCRIPTIONS
Frame Style Transparent Dressing are designed to allow oxygen and moisture vapor exchange, jet provide a barrier to resist liquids and bacteria. They are comformable, and flex with skin for patient comfort.
INDICATIONS FOR USE
Frame Style Transparent Dressing are indicated for I.V. sites, surgical incise, primary dressing on pressure ulcers (stages I and II) with minimal drainage, partial thickness wounds, and skin tears or abrasions. They are also used to aid in autolylic debridement.
CONTRAINDICATIONS
Frame Style Transparent Dressing are contraindicated as a primary dressing on moderately to heavily draining wounds. Avoid repeated applications on patients with thin or fragile skin that may result in skin damage.
APPLICATION
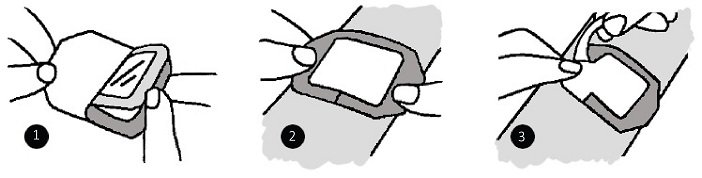
1. Prepare the wound or site according to facility protocol. Allow all skin cleansing and preparation solutions to dry completely.
2. Remove dressing from package. Peel back lining from the dressing.
3. position the dressing ove the wound or site and apply using light pressure. Do not stretch the dressing while applying.
4. Gently remove the frame, smoothing the dressing down as the frame is being pulled away.
5. Smooth the dressing from the center toward the edges to aid adhesion.
6. Date and initial the dressing in the spaces provided.
REMOVAL
1. Change dressing according to standard wound care practices and facility protocol.
2. Supporting the skin, gently lift corner and slowly peel the dressing from the skin in the direction of the hair growth. Peel the dressing back, parallel to the skin, rather than pulling it up from the skin.
3. Dispose of the dressing according to facility protocol.
PRECAUTIONS FOR USE
1. To assure good adhesion, the skin should be clean, dry and free from skin oils, soaps, detergents and lotions. Ensure that no wet skin preparationsolution or soap residues are trapped under the dressing.
2. The dressing should be allowed a minimum overlap of 1 inch (3 cm) from the margin of the wound onto the surrounding healthy skin.
3. Removed excess hair from around the site, as required, by clipping not shaving. Do not reuse.
STORAGE: Avoid storage in direct sunlight/flourescent lighting and keep area cool, dry, and well-ventilated.
Contents STERILE in unopened, undamaged inner package. Not made with natural rubber latex.
| LIDOTOR
lidocaine and prilocaine kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PureTek Corporation (785961046) |
More about Lidotor (lidocaine / prilocaine topical)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical anesthetics
Professional resources
Other brands
Emla, Lidopril XR, Dolotranz, Oraqix, ... +22 more

