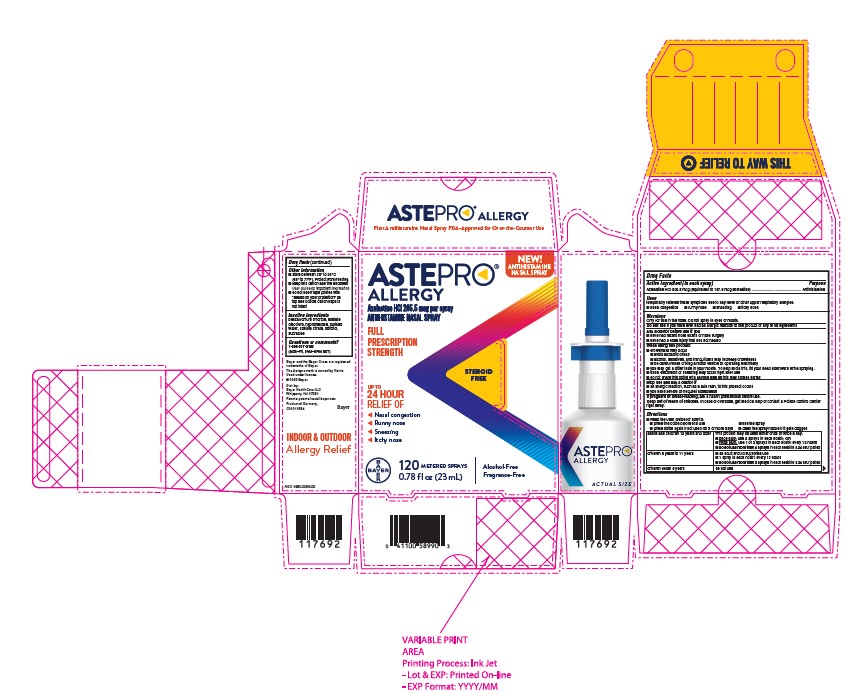
Astepro Allergy Prescribing Information
Package insert / product label
Generic name: azelastine hydrochloride
Dosage form: nasal spray, metered
Drug class: Nasal antihistamines and decongestants
Medically reviewed by Drugs.com. Last updated on Sep 27, 2023.
On This Page
Indications and Usage for Astepro Allergy
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- nasal congestion
- runny nose
- sneezing
- itchy nose
Warnings
Only for use in the nose. Do not spray in eyes or mouth.
Ask a doctor before use if you
- have had recent nose ulcers or nose surgery
- have had a nose injury that has not healed
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- you may get a bitter taste in your mouth. To help avoid this, tilt your head downward while spraying.
- nasal discomfort or sneezing may occur right after use
- do not share this bottle with anyone else as this may spread germs
Astepro Allergy Dosage and Administration
Read the User Guide for how to:
- prime the bottle before first use
- prime bottle again if not used for 3 or more days
- use the spray
- clean the spray nozzle if it gets clogged
| adults and children 12 years and older |
This product may be used either once or twice a day:
|
|
children 6 years to 11 years |
|
| children under 6 years | do not use |
Other information
Other information
- store between 20° to 25° C (68° to 77° F). Protect from freezing.
- keep this carton and the enclosed User Guide for important information
- do not use if tape printed with “sealed for your protection” on top and bottom carton flaps is not intact
| ASTEPRO ALLERGY
azelastine hcl spray, metered |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
More about Astepro Allergy (azelastine nasal)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: nasal antihistamines and decongestants
- Breastfeeding
- En español