AK-POLY-BAC Prescribing Information
Package insert / product label
Generic name: bacitracin zinc and polymyxin b sulfate
Dosage form: ophthalmic ointment
Drug class: Ophthalmic anti-infectives
Medically reviewed by Drugs.com. Last updated on Jan 15, 2024.
On This Page
AK-POLY-BAC Description
Bacitracin Zinc and Polymyxin B Sulfate Ophthalmic Ointment is a sterile antimicrobial ointment for ophthalmic use.
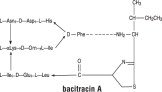
Bacitracin zinc is the zinc salt of bacitracin, a mixture of related cyclic polypeptides (mainly bacitracin A) produced by the growth of an organism of the licheniformis group of Bacillus subtilis var Tracy. It has a potency of not less than 40 bacitracin units per mg. The structural formula for bacitracin A is:
Polymyxin B Sulfate is the sulfate salt of polymyxin B1 and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on an anhydrous basis. The structural formulae are:
Each gram contains: Bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units, white petrolatum and mineral oil.
AK-POLY-BAC - Clinical Pharmacology
Polymyxin B attacks gram-negative bacilli, including virtually all strains of Pseudomonas aeruginosa and H influenzae species.
Bacitracin is active against most gram-positive bacilli and cocci including hemolytic streptococci.
Indications and Usage for AK-POLY-BAC
For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to bacitracin zinc and polymyxin B sulfate.
Contraindications
This product is contraindicated in those individuals who have shown hypersensitivity to any of its components.
Precautions
As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. Appropriate measures should be taken if this occurs.
AK-POLY-BAC Dosage and Administration
Apply the ointment every 3 or 4 hours for 7 to 10 days, depending on the severity of the infection.
How is AK-POLY-BAC supplied
Bacitracin Zinc and Polymyxin B Sulfate ophthalmic ointment USP, sterile, each gram contains bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units, in a tube of 3.5 g (1/8 oz) with ophthalmic tip.
NDC 17478-238-35
Storage and Handling
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Akorn
Manufactured By: Akorn, Inc.
Lake Forest, IL 60045
AKP00N
Rev. 06/16
| AK-POLY-BAC
bacitracin zinc and polymyxin b sulfate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akorn (117696770) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696840 | MANUFACTURE(17478-238) , ANALYSIS(17478-238) , STERILIZE(17478-238) , PACK(17478-238) , LABEL(17478-238) | |
Frequently asked questions
More about AK-Poly-Bac (bacitracin / polymyxin b ophthalmic)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: ophthalmic anti-infectives