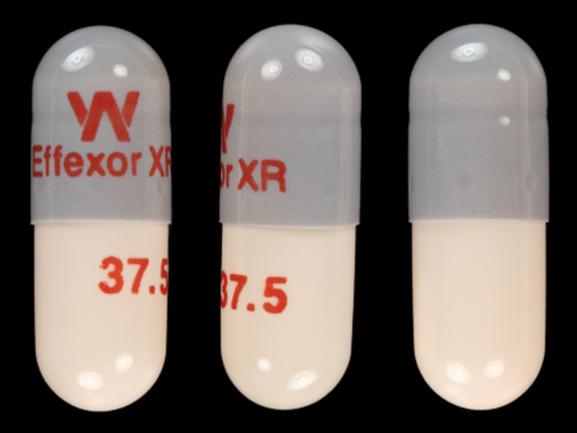
W Effexor XR 37.5 Pill - gray & peach capsule/oblong, 16mm
Generic Name: venlafaxine
Pill with imprint W Effexor XR 37.5 is Gray & Peach, Capsule/Oblong and has been identified as Effexor XR 37.5 mg. It is supplied by Wyeth-Ayerst Laboratories.
Effexor XR is used in the treatment of Autism; Major Depressive Disorder; Anxiety; Depression; Generalized Anxiety Disorder and belongs to the drug class serotonin-norepinephrine reuptake inhibitors. Risk cannot be ruled out during pregnancy. Effexor XR 37.5 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for W Effexor XR 37.5
Effexor XR
- Generic Name
- venlafaxine
- Imprint
- W Effexor XR 37.5
- Strength
- 37.5 mg
- Color
- Gray & Peach
- Size
- 16.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Serotonin-norepinephrine reuptake inhibitors
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Wyeth-Ayerst Laboratories
- Inactive Ingredients
-
cellulose powdered,
ferric oxide red,
titanium dioxide,
ethylcellulose (100 mPa.s),
hypromellose 2910 (6 mPa.s)
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00008-0837 | Wyeth |
| 60505-3778 (Discontinued) | Apotex Corp. |
| 59762-0180 (Discontinued) | Viatris |
| 54868-4504 (Discontinued) | Physicians Total Care Inc. (repackager) |
| 52959-0771 | H.J. Harkins Company, Inc. (repackager) |
| 33358-0124 (Discontinued) | CorePharma, LLC (repackager) |
| 68071-0333 | Nucare Pharmaceuticals Inc. (repackager) |
Related images for "W Effexor XR 37.5"
More about Effexor XR (venlafaxine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (997)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Support group
- Drug class: serotonin-norepinephrine reuptake inhibitors
- Breastfeeding
- En español
Patient resources
- Effexor XR drug information
- Effexor-XR (Advanced Reading)
- Effexor XR (Venlafaxine Extended-Release Capsules)
- Effexor XR (Venlafaxine Extended-Release Tablets)
Professional resources
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

