93 T13 Pill - tan & white capsule/oblong, 19mm
Pill with imprint 93 T13 is Tan & White, Capsule/Oblong and has been identified as Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride Extended Release 60 mg / 120 mg. It is supplied by Teva Pharmaceuticals USA.
Fexofenadine/pseudoephedrine is used in the treatment of Allergic Rhinitis; Allergies and belongs to the drug class upper respiratory combinations. Risk cannot be ruled out during pregnancy. Fexofenadine/pseudoephedrine 60 mg / 120 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for 93 T13
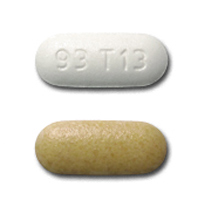
Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride Extended Release
- Imprint
- 93 T13
- Strength
- 60 mg / 120 mg
- Color
- Tan & White
- Size
- 19.00 mm
- Shape
- Capsule/Oblong
- Availability
- Rx and/or OTC
- Drug Class
- Upper respiratory combinations
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Teva Pharmaceuticals USA
- National Drug Code (NDC)
- 00093-1130 (Discontinued)
Related images for "93 T13"
More about fexofenadine / pseudoephedrine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (63)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: upper respiratory combinations
Patient resources
Other brands
Allegra-D 24 Hour, Allegra-D 12 Hour, Allegra-D 12 Hour Allergy & Congestion
Professional resources
Other brands
Allegra-D 24 Hour, Allegra-D 12 Hour
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

